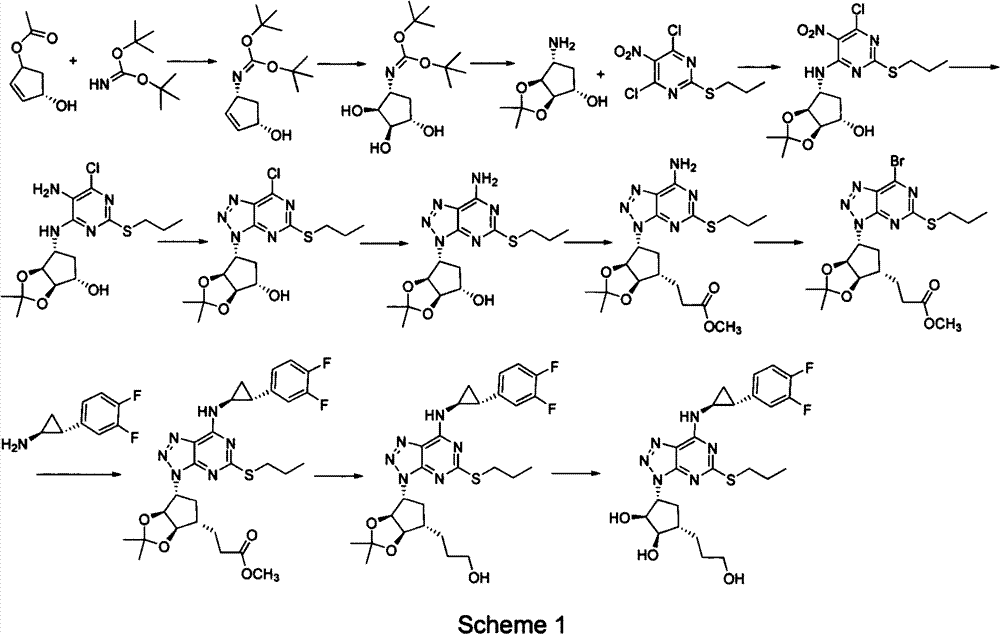
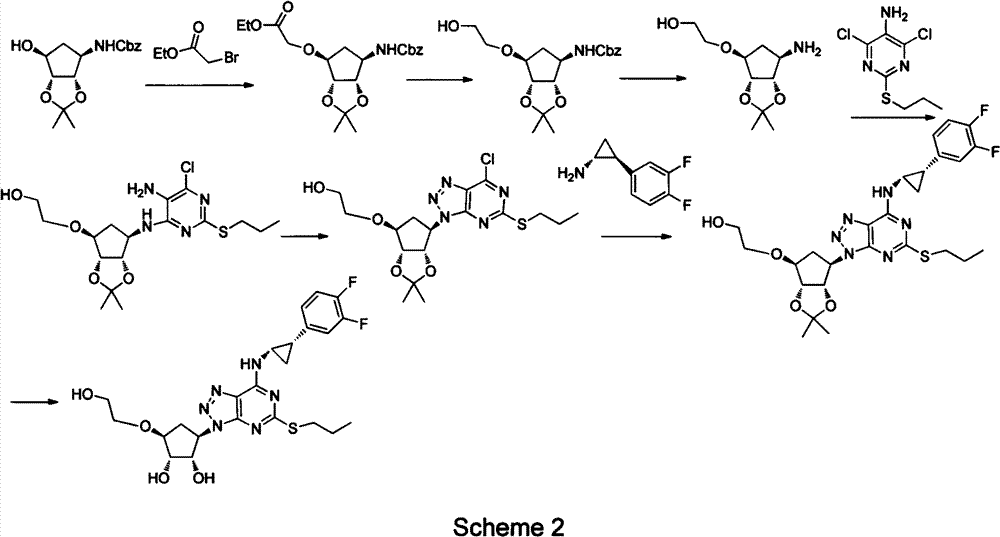
Preparation method of ticagrelor
A technology for ticagrelor and compounds, applied in the field of preparation of ticagrelor, capable of solving problems such as long route, low total yield, and unsuitability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Compound Via
[0025]
[0026] Compound VIIa (615 g, 2 mol) was added into 3.5 L of tetrahydrofuran, and potassium tert-butoxide solid (269.5 g, 2.4 mol) was added in portions. The mixture was stirred for 10 minutes, and a 1L tetrahydrofuran solution of 2-bromoethoxy tert-butyldimethylsilane (573.5g, 2.4mol) was added dropwise at 0°C. The reaction solution was stirred for 1.5 hours. TLC showed that the reaction was complete, and 2L of water was added to the reaction solution. , ethyl acetate 2L, the organic phase was separated, dried, and concentrated to obtain compound VIa857.3g, with a yield of 92%.
[0027] Preparation of compound Va
[0028]
[0029] Compound VIa (838.2g, 1.8mol) was added to 5L of ethanol, 10% palladium carbon (40g) was added, the reaction solution was replaced with hydrogen to remove air, pressurized at 1.5 Pa, and reacted at 50°C for 10 hours. TLC showed that the reaction was complete. Palladium carbon was removed by filtra...
Embodiment 2
[0043] Preparation of compound VIb
[0044]
[0045] Compound VIIb (615 g, 2 mol) was added to 3 L of N,N-dimethylformamide, and 40% solid sodium hydride (144 g, 2.4 mol) was added in portions. The mixture was stirred for 10 minutes, and 2-iodoethoxytriethylsilane (687g, 2.4mol) in 1L N,N-dimethylformamide solution was added dropwise at zero degree, and the reaction solution was stirred for 1 hour, TLC showed that the reaction was complete, and the reaction 2 L of water and 2 L of ethyl acetate were added to the solution, the organic phase was separated, dried and concentrated to obtain 47.5 g of compound VIb with a yield of 91%.
[0046] Preparation of Compound Vb
[0047]
[0048] Compound VIb (838.2g, 1.8mol) was added to 5L of methanol, 15% palladium hydroxide (40g) was added, the reaction solution was replaced with hydrogen to remove air, pressurized at 2 Pa, and reacted at 50°C for 12 hours. TLC showed that the reaction was complete. The liquid was filtered to remo...
Embodiment 3
[0062] Preparation of Compound VIc
[0063]
[0064] Compound VIIc (615 g, 2 mol) was added to 5 L of methyl tert-butyl ether, and sodium tert-amylate solid (264 g, 2.4 mol) was added. The mixture was stirred for 10 minutes, and a 1L solution of 1-chloro-2-methoxymethylethane (299 g, 2.4 mol) was added dropwise at zero degree, and the reaction solution was stirred for 1 hour. TLC showed that the reaction was complete, and the reaction solution 2 L of water and 2 L of ethyl acetate were added, and the organic phase was separated, dried, and concentrated to obtain 704.9 g of compound VIc with a yield of 89%.
[0065] Preparation of compound Vc
[0066]
[0067] Compound VIc (672.3g, 1.7mol) was added to 4L of isopropanol, 10% palladium carbon (30g) was added, the reaction solution was replaced with hydrogen to remove air, pressurized at 1.5Pa, and reacted at 50°C for 12 hours. TLC showed that the reaction was complete. The reaction solution was filtered to remove palladi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com