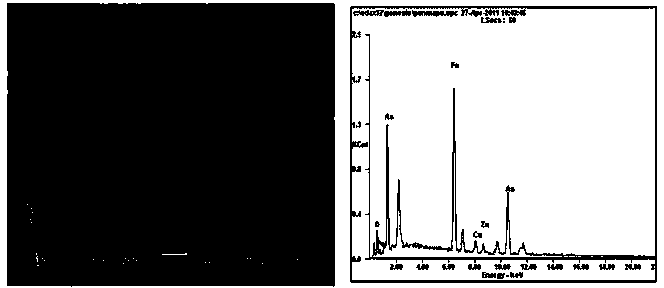
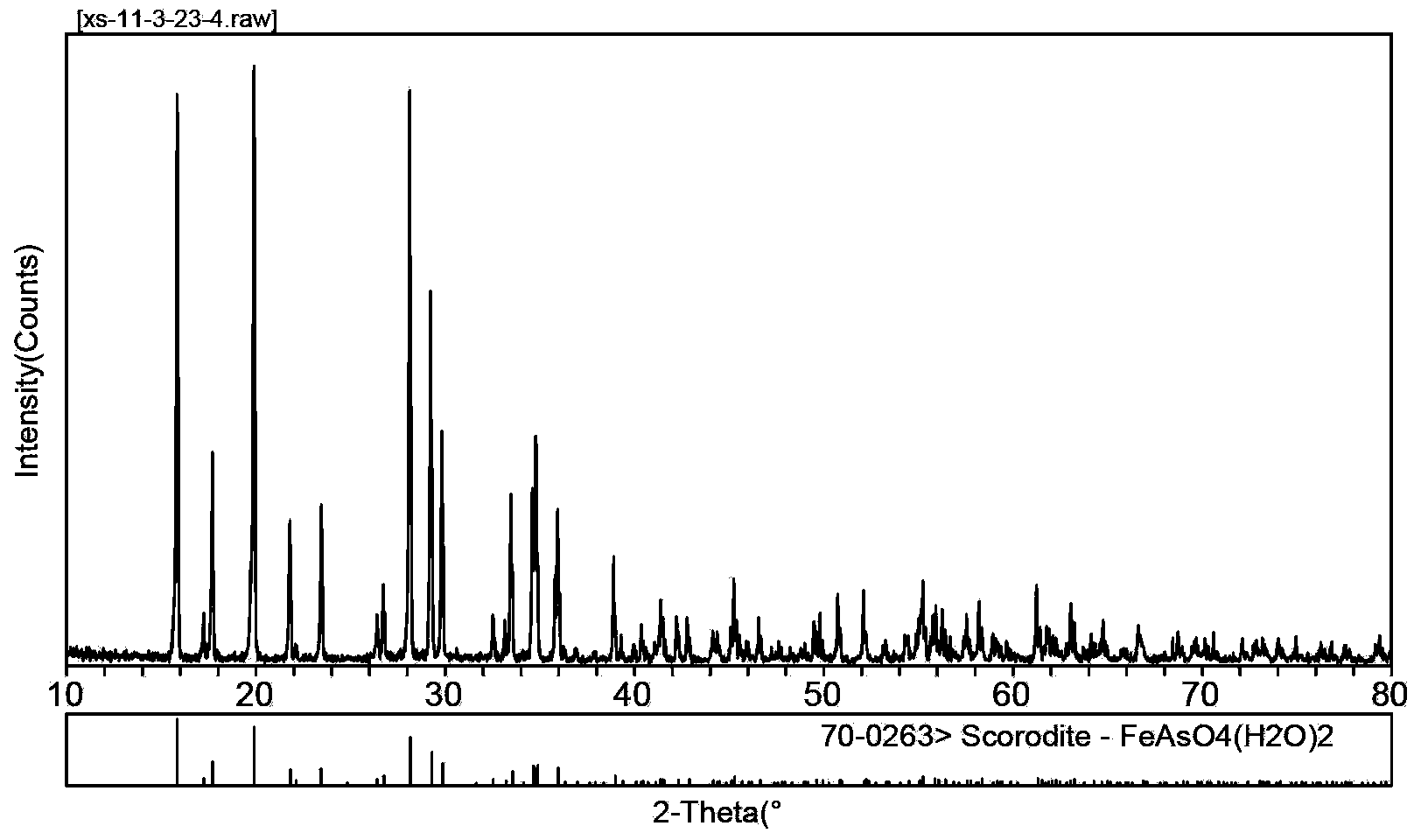
Method for precipitating and stabilizing As from As-containing solution
A technology of solution and arsenic precipitation, which is applied in chemical instruments and methods, water pollutants, water/sludge/sewage treatment, etc., can solve the problems of harsh experimental conditions and achieve good crystal structure, low cost, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 The arsenic solution (pH=4) with a concentration of 10 g / L precipitates arsenic to form iron arsenate crystals.
[0029] Add 16.11g of crude sodium arsenate crystals into 250ml of distilled water, stir to dissolve, add 95% concentrated sulfuric acid drop by drop and measure the pH, adjust the pH to 4, add the prepared arsenic-containing solution into a 1000ml three-necked flask, heat in a water bath and Stir mechanically, set the temperature to 95° C., and the stirring speed to 200 rpm. Another 27.80g FeSO 4 ·7H 2 O was added to 250ml of distilled water, stirred to dissolve, and added to the preheated arsenic-containing solution. Under this condition, the molar mass ratio of iron to arsenic is 1.5, and the initial arsenic concentration is 10g / L. Continue heating in a water bath to 95°C with stirring, blow in preheated air and time the reaction for 5 hours, and control the air flow rate to 120 L / h. After the reaction, the solution was cooled and allowed to ...
Embodiment 2
[0031] Embodiment 2 concentration is that 50g / L arsenic-containing solution synthesizes iron arsenate crystal
[0032] Add 80.54g of crude sodium arsenate crystals to 250ml of distilled water, stir to dissolve, add 95% concentrated sulfuric acid drop by drop and measure the pH, adjust the pH to 4, add the prepared arsenic-containing solution into a 1000ml three-necked flask, heat in a water bath and Mechanical stirring, the stirring speed is 200rpm. Take 139.01g FeSO 4 ·7H 2 O was added to 250ml of distilled water, stirred to dissolve, and slowly added to the preheated arsenic-containing solution. Under this condition, the molar mass ratio of iron to arsenic is 1.5, and the initial arsenic concentration is 50g / L. Continue heating to 95°C in a water bath with stirring, blow in preheated air and time the reaction for 7 hours, and control the air flow rate to 120 L / h. After the reaction, the reaction solution was cooled and allowed to stand, and the temperature was slightly l...
Embodiment 3
[0033] Example 3 Synthesis of ferric arsenate crystals under different reaction temperature conditions
[0034] Add 16.11g of crude sodium arsenate crystals into 250ml of distilled water, stir to dissolve, add 95% concentrated sulfuric acid drop by drop and measure the pH, adjust the pH to 4, add the prepared arsenic-containing solution into a 1000ml three-necked flask, heat in a water bath and Mechanical stirring, the stirring speed is 200rpm. Take 27.80g FeSO 4 ·7H 2O was added to 250ml of distilled water, stirred to dissolve, and added to the preheated arsenic-containing solution. Continue to heat in a water bath to 70, 80, and 95°C with stirring, blow in preheated air and time the reaction for 7 hours, and control the air flow rate to 120L / h. After the reaction, the reaction solution was cooled and left to stand, and the temperature was slightly lowered to 60 ° C. The solution was filtered while it was hot, and the sediment was washed once with 100 ml of distilled water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com