Preparation method of flower-like alpha-nickel hydroxide
A nickel hydroxide and flower-like technology, which is applied in the field of preparation of inorganic compounds, can solve the problems of few pure-phase flower-like α-Ni
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
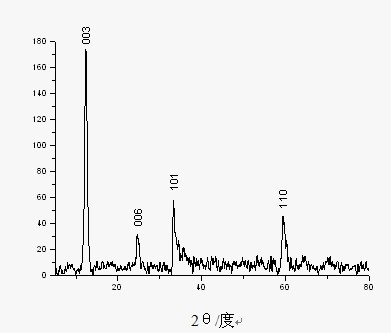
[0024] Add 0.238 g of nickel chloride hexahydrate and 0.280 g of urotropine into 5 mL of deionized water and stir to dissolve, then add 25 mL of ethanol to the mixture; add deionized water to the resulting solution to a volume of 40 mL, transferred to a 50 mL polytetrafluoroethylene-lined autoclave, reacted at 160 °C for 2 h, cooled, filtered, and washed several times with deionized hydrated absolute ethanol to filter out the product, and Placed in a vacuum drying oven at 60 °C to obtain flower-shaped α-Ni(OH) 2 . Its XRD image see figure 1 , SEM image see figure 2 .
[0025]
Embodiment 2
[0027] Add 1.45 g of nickel nitrate hexahydrate and 0.70 g of urotropine into 5 mL of deionized water and stir to dissolve, then add 10 mL of methanol to the mixture; add deionized water to the resulting solution to a volume of 40 mL, transferred to a 50 mL polytetrafluoroethylene-lined autoclave, reacted at 160 °C for 2 h, cooled, filtered, and then washed several times with deionized hydrated absolute ethanol to filter out the product, and placed Dry in a vacuum oven at 60 °C to obtain flower-like α-Ni(OH) 2 . Its XRD image and SEM image are as embodiment 1.
[0028]
Embodiment 3
[0030] Add 0.131 g of nickel sulfate hexahydrate and 0.210 g of urotropine into 5 mL of deionized water and stir to dissolve, then add 35 mL of ethanol to the mixture; add deionized water to the resulting solution to a volume of 40 mL, transferred to a 50 mL polytetrafluoroethylene-lined autoclave, reacted at 160 °C for 2 h, cooled, filtered, and then washed several times with deionized hydrated absolute ethanol to filter out the product, and placed Dry in a vacuum oven at 60 °C to obtain flower-like α-Ni(OH) 2 . Its XRD image and SEM image are as embodiment 1.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com