Preparation method of barium carbonate and product prepared by same
A technology of barium carbonate and products, which is applied in the direction of barium carbonate, calcium carbonate/strontium/barium, thin material treatment, etc., can solve the problems of large influence of impurities, lack of research, and difficult cleaning, etc., and achieve the effect of improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
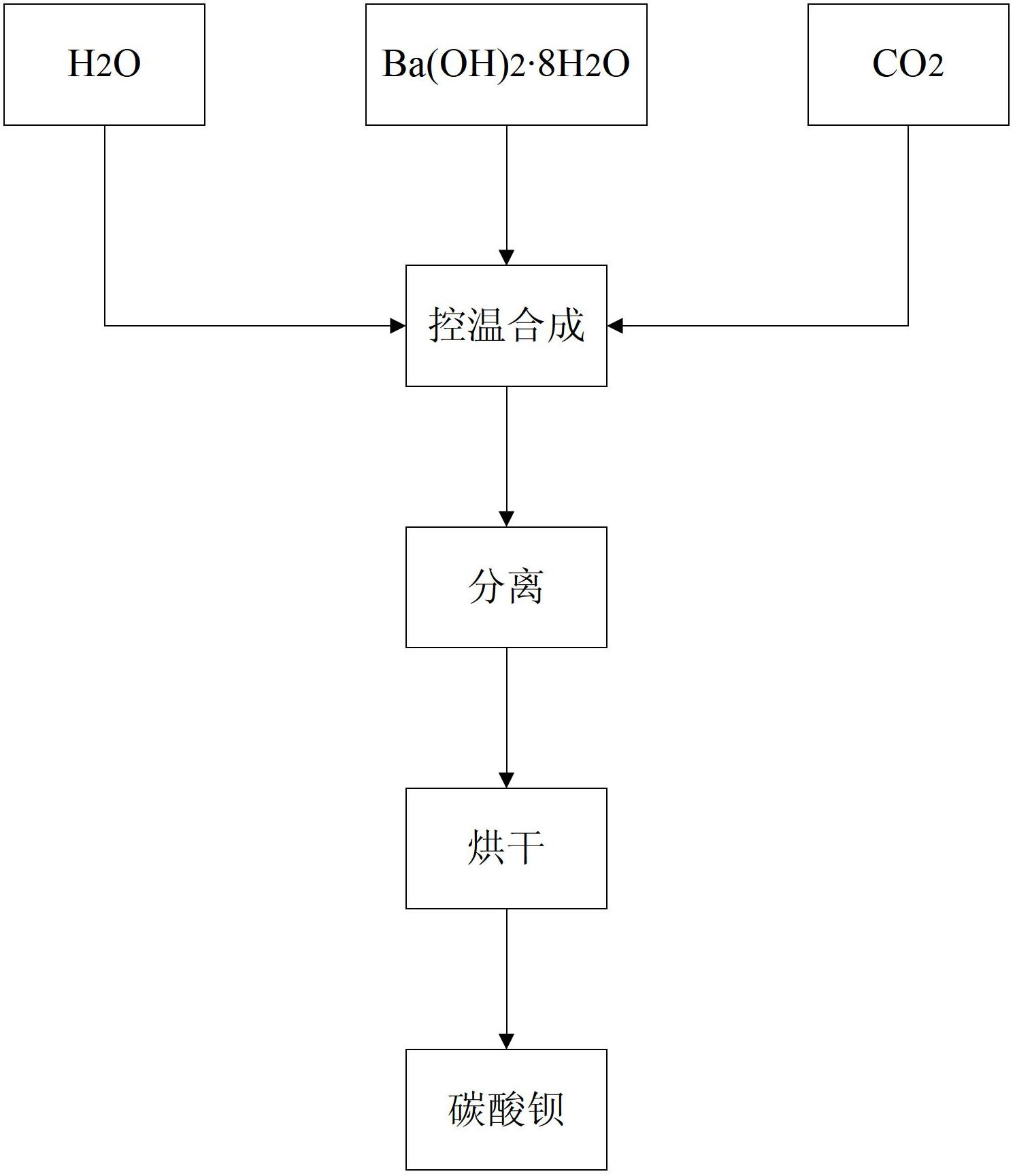
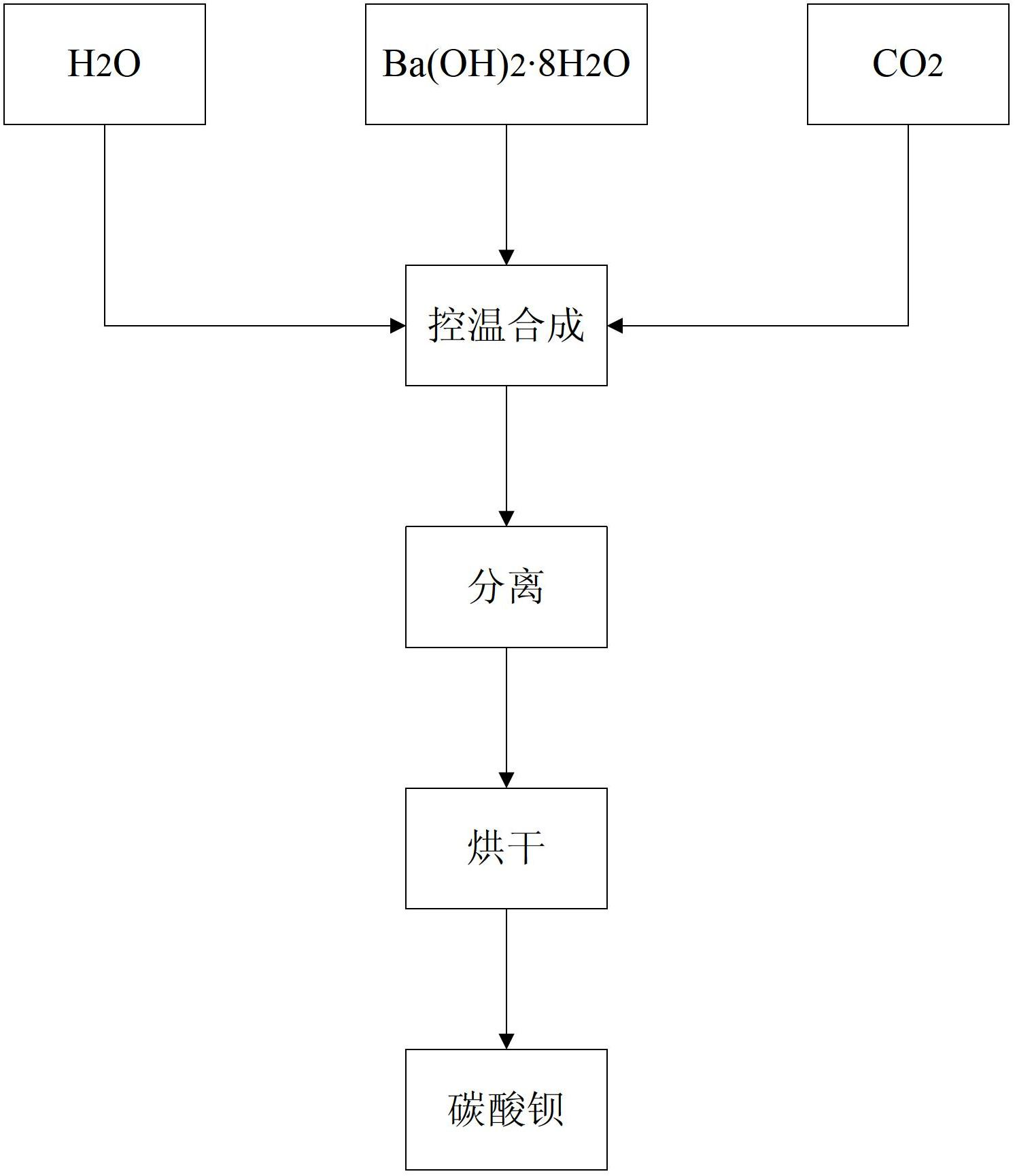
[0053] Put 4000ml of deionized water in a 5000ml beaker, cool in an ice-water bath under stirring, control the temperature at 4°C, and add high-purity Ba(OH) to be reacted 2 ·8H 2 O crystal (purity greater than 98%, strontium content less than 10ppm) 321.4g, after stirring for 5 minutes, insert CO 2 Gas bubbling absorption reaction, controlling CO 2 The gas flow rate is 250ml / min until the pH of the reaction solution is 6.5, the solid and liquid are separated, the filtrate is discarded, and the solid is dried in a vacuum oven at 60°C for 24 hours to obtain a high-purity barium carbonate sample with low strontium and large specific surface area 3 # .
Embodiment 2
[0055] Put 4000ml of deionized water in a 5000ml beaker, cool in an ice-water bath under stirring, control the temperature at 5°C, and add high-purity Ba(OH) to be reacted 2 ·8H 2 O crystal (purity greater than 98%, strontium content less than 10ppm) 385.7g, after stirring for 5 minutes, insert CO 2 Gas bubbling absorption reaction, controlling CO 2 The gas flow rate is 270ml / min until the pH of the reaction solution is 6.5, the solid and liquid are separated, the filtrate is discarded, and the solid is dried in a vacuum oven at 60°C for 24 hours to obtain a high-purity barium carbonate sample with low strontium and large specific surface area 4 # .
Embodiment 3
[0057] Put 4000ml of deionized water in a 5000ml beaker, cool in an ice-water bath under stirring, control the temperature at 4°C, and add high-purity Ba(OH) to be reacted 2 ·8H 2 O crystal (purity greater than 98%, strontium content less than 10ppm) 450g, after stirring for 5 minutes, insert CO 2 Gas bubbling absorption reaction, controlling CO 2 The gas flow rate is in the range of 270ml / min until the pH of the reaction solution is 7.0, the solid and liquid are separated, the filtrate is discarded, and the solid is dried in a vacuum oven at 60°C for 24 hours to obtain a high-purity barium carbonate sample with low strontium and large specific surface area 5 # .
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com