Process for production of glycidyl ether compounds, and monoallyl monoglycidyl ether compound
A technology of glycidyl ether and manufacturing method, applied in the directions of metal/metal oxide/metal hydroxide catalyst, chemical instrument and method, chemical/physical process, etc., can solve the problem of insufficient yield, inapplicability, expensive catalyst and oxidizing agents, to achieve the effects of simple operation, safety, inhibition of impurity mixing, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
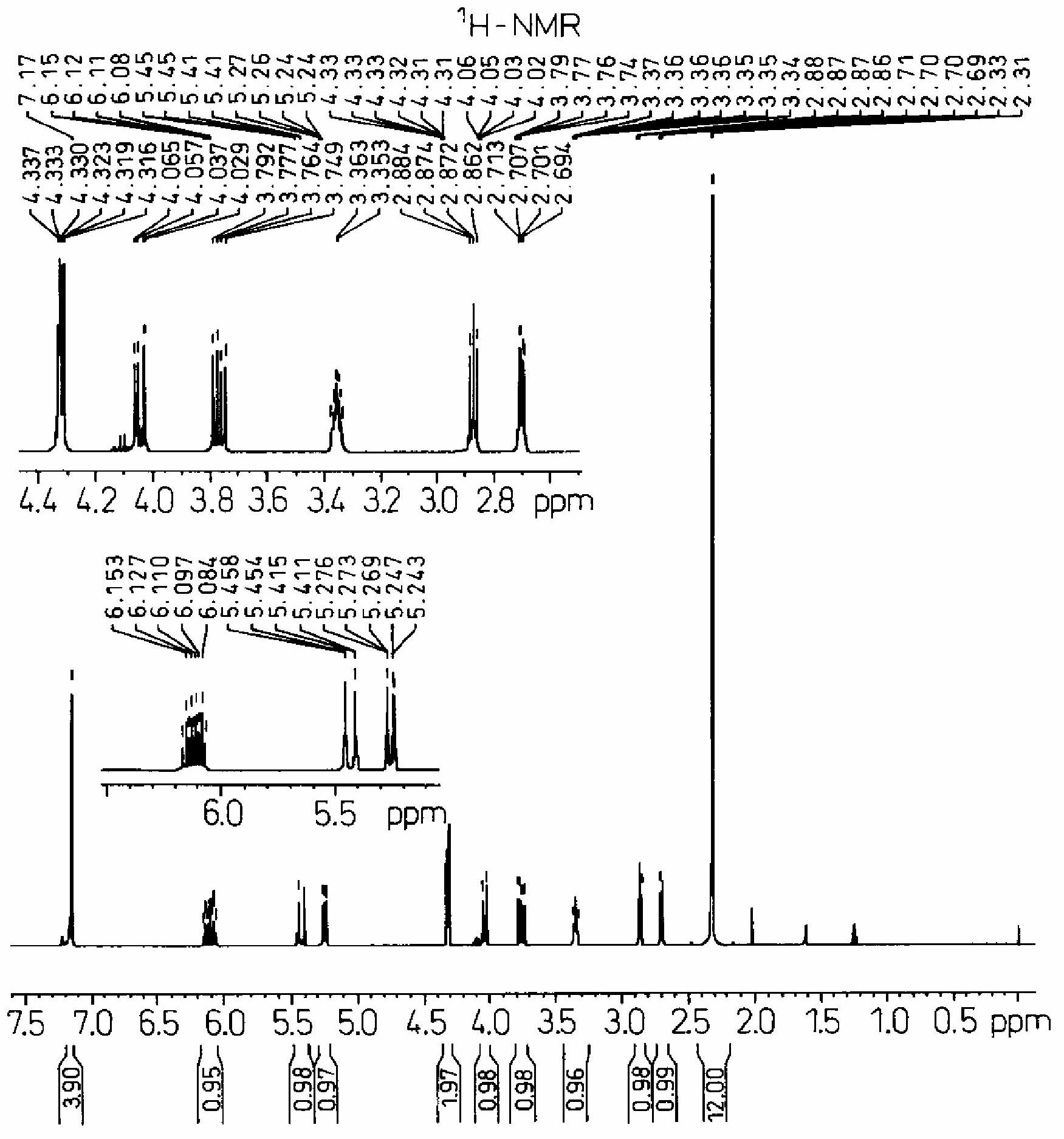
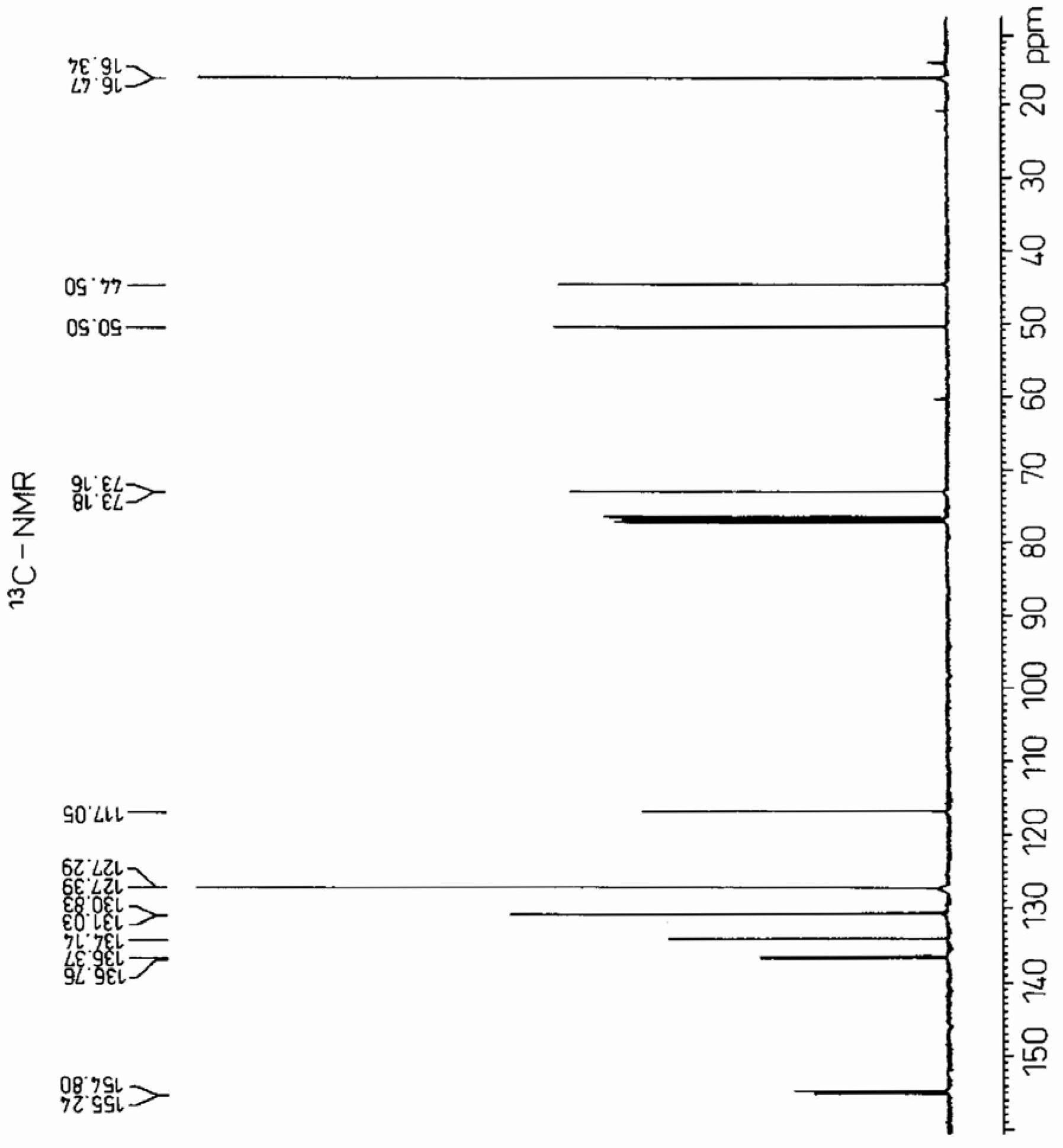
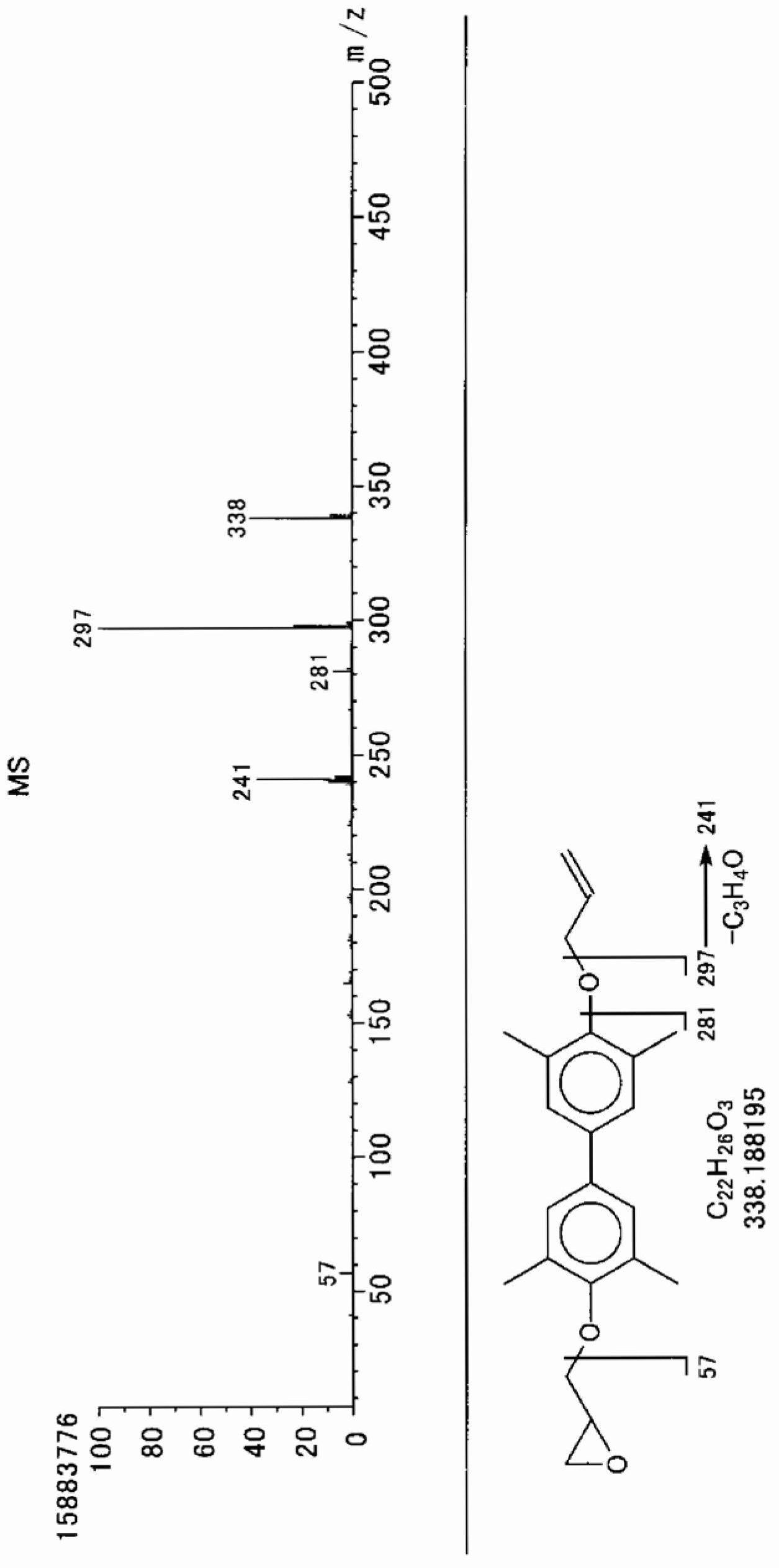
[0072] Add 0.950 g (2.88 mmol) of sodium tungstate (manufactured by Japan Inorganic Chemical Industry Co., Ltd.), tungstic acid (manufactured by Japan Inorganic Chemical Industry Co., Ltd.) ) 0.720g (2.88mmol), trioctylamine (manufactured by Koei Chemical Co., Ltd.) 2.04g (5.76mmol), phenylphosphonic acid (manufactured by Nissan Chemical Co., Ltd.) 0.911g (5.76mmol), olefin Propyl phenyl ether 80g (0.576 moles), while stirring with an electromagnetic stirrer, after heating to 70°C with an oil bath, 84.0g of 35% hydrogen peroxide aqueous solution was added dropwise in a manner that the reaction temperature did not exceed 75°C ( 0.864 mol). After the dropwise addition, stirring was continued for 2 hours, and the reaction solution was cooled to room temperature. Then, 40 g of ethyl acetate was added, the organic layer was brought to the upper layer, the water layer was made to come to the lower layer, and the organic layer was separated.
[0073] As a result of analyzing the or...
Embodiment 2~10
[0082] The epoxidation reaction was performed in the same manner as in Example 1 using the catalyst components and molar ratios shown in Table 1 below. The results are collectively shown in Table 1 below.
[0083] [Table 1]
[0084]
Synthetic example 1
[0085] [Synthesis Example 1]: Synthesis of Diallyl Ether of Bisphenol F
[0086] Add 200 g (0.999 mol) of bisphenol F-ST (manufactured by Mitsui Chemicals Co., Ltd.) and 50% water-containing 5%-Pd / C-STD type (manufactured by Eno Ichem Kitatsuto Co., Ltd.) to a 2000 ml eggplant-shaped flask 2.13g (0.499mmol), triphenylphosphine (manufactured by Hokko Chemical Co., Ltd.) 2.62g (9.99mmol), potassium carbonate (manufactured by Asahi Glass Co., Ltd.) 276g (2.00mol), allyl acetate (Showa Denko Co., Ltd.) 220 g (2.20 mol) and 200 g of isopropanol were reacted at 85° C. for 8 hours in a nitrogen atmosphere. After the reaction, a part of the sample was sampled and diluted with ethyl acetate, and gas chromatography analysis confirmed that the ratio of bisphenol F diallyl ether and monoallyl ether was 99:1.
[0087] Then, 400 g of toluene was added to the reaction liquid, Pd / C and the precipitated solid were removed by filtration, and isopropanol and toluene were distilled off by an eva...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com