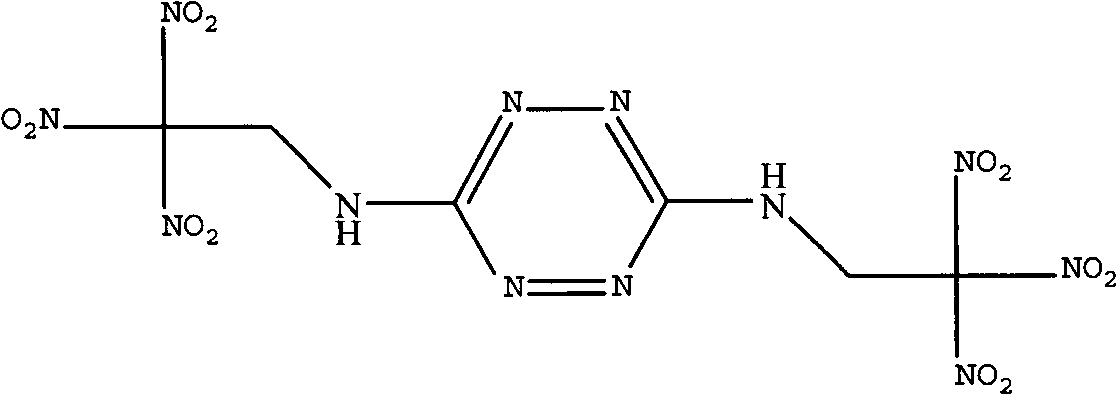
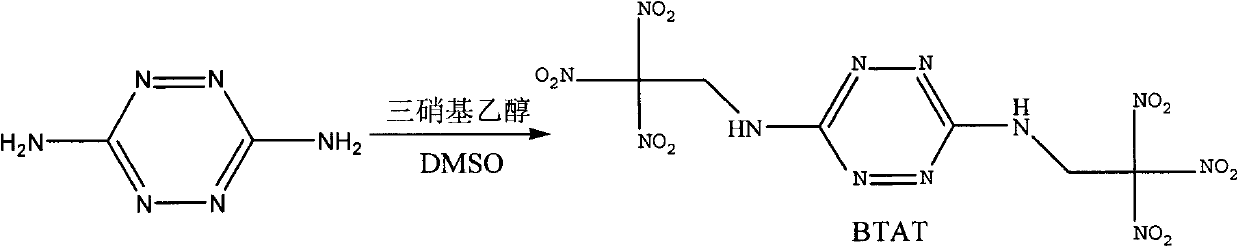
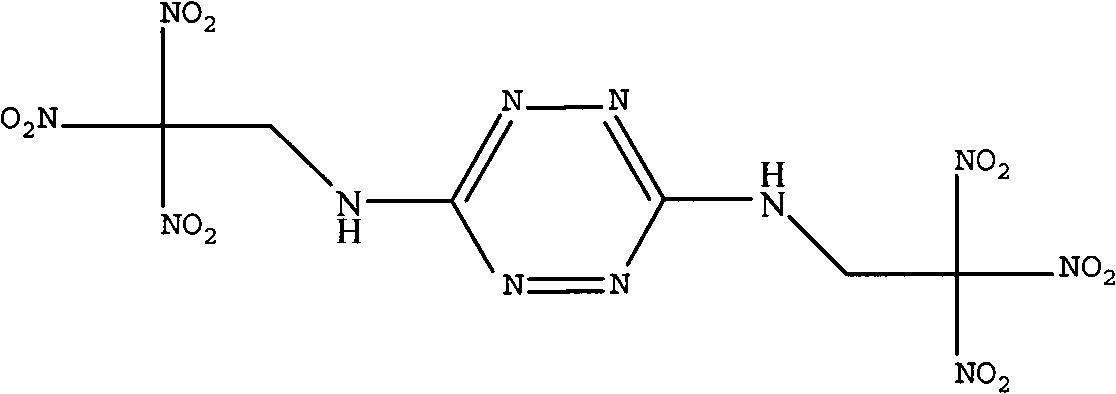
Synthetic method of bis(2,2,2-trinitro ethyl)-3-6-diamino tetrazine
A technology of trinitroethyl and diaminotetrazine, applied in the direction of organic chemistry, etc., can solve the problems of unspecified reaction yield and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] Preparation of trinitroethanol:
[0018] Under an ice-water bath, add 33.6g (0.3mol) of 4,6-dihydroxypyrimidine to concentrated sulfuric acid in batches, and after it is completely dissolved, add 96.3g (1.5mol) of concentrated nitric acid dropwise, react at 42°C for 2h, and pour the reaction solution into Put into ice water, extract with dichloromethane, dry over anhydrous magnesium sulfate, and distill off dichloromethane under atmospheric pressure to obtain trinitromethane solution containing a small amount of dichloromethane.
[0019] Add 15 g of the above-mentioned trinitromethane solution containing dichloromethane into carbon tetrachloride, then add paraformaldehyde, and stir under reflux for 3 h. Dichloromethane and part of carbon tetrachloride were distilled off under reduced pressure, cooled, filtered, and dried to obtain 12.4 g of trinitroethanol.
Embodiment 1
[0021] Dissolve 11.2g (0.1mol) of 3,6-diaminotetrazine in 200mL of dimethyl sulfoxide, and add the above solution to 180.9g of 22% (mass) trinitrate under stirring at 0°C to 5°C In the aqueous solution of base ethanol, and while adding the aqueous solution of trinitroethanol, adjust the pH of the reaction system to 2 with sulfuric acid, after adding the dimethyl sulfoxide solution of 3,6-diaminotetrazine, the After reacting at ℃ for 3 hours, the reaction solution was poured into 300 mL of ice water, and a large amount of solids were precipitated. After filtering and drying the filter cake, 27.4 g of BTAT was obtained with a yield of 62.6%.
[0022] Structure Identification:
[0023] Infrared (KBr, cm -1 ): 3285 (-NH-), 1612, 1587 (tetrazine ring), 1532, 1313 (-NO 2 ), 2964 (-CH 2 -).
[0024] NMR (DMSO, δ, ppm): 6.8(-NH-), 2.5(-CH 2 -).
[0025] Mass spectrum: 438[m, 13.5%], 392[m-NO 2 , 1.4%], 346[m-2NO 2 , 1.5%], 300[m-3NO 2 , 2.0%], 206[N-C-NH-CH 2 -C(NO 2 ) 3 ...
Embodiment 2
[0031] Dissolve 11.2g (0.1mol) of 3,6-diaminotetrazine in 200mL of dimethyl sulfoxide, add the above solution to 181g of trinitroethanol with a mass fraction of 20% under stirring at 0°C to 5°C In the aqueous solution, the pH of the reaction system can be kept at 1 with 36% hydrochloric acid during the dropping process. After adding the dimethyl sulfoxide solution of 3,6-diaminotetrazine, react at 20°C to 25°C for 2 hours , the reaction solution was poured into 500 mL of ice water, a large amount of solids were precipitated, filtered and dried to obtain 22.7 g of the target compound BTAT, with a yield of 51.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com