Method for preparing 4,6-dichloro-5-amino-2-(propylsulfanyl)pyrimidine
A technology of propylthiopyrimidine and mercaptopyrimidine, which is applied in the field of preparation of 4,6-dichloro-5-amino-2-propylthiopyrimidine, can solve the problems of complicated product post-processing, high reaction temperature, reduced yield, etc. problems, to achieve the effect of shortening the preparation period, lowering the reaction temperature, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
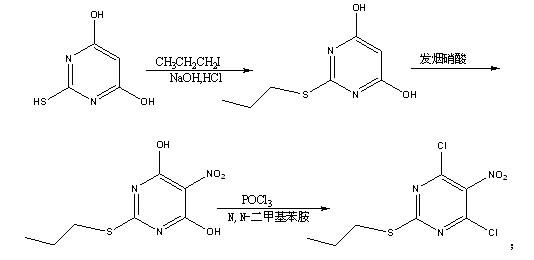
[0021] A kind of preparation method of 4,6-dichloro-5-amino-2-propylthiopyrimidine of the present invention, comprises the steps:
[0022] Add 4,6-dihydroxy-2-mercaptopyrimidine into water, add dropwise 10% sodium hydroxide solution to dissolve, then add methanol and 1-bromopropane, keep the reaction at 40-45°C for 15-20 hours, and cool to At room temperature, acidify and crystallize to obtain 4,6-dihydroxy-2-propylthiopyrimidine, the mass ratio of sodium hydroxide solution to methanol is 1:1, the mass of 4,6-dihydroxy-2-mercaptopyrimidine to water The ratio is 0.14: the mass ratio of 2,4,6-dihydroxy-2-mercaptopyrimidine to 1-bromopropane is 1:1.7-1.8;
[0023] Compound I; Compound II;
[0024] Add fuming nitric acid to glacial acetic acid, add 4,6-dihydroxy-2-propylthiopyrimidine at low temperature, after the reaction is completed, add water to crystallize to obtain 4,6-dihydroxy-5-nitro-2-propane The mass ratio of thiopyrimidine, 4,6-dihydroxy-2-propylthiopyrimidin...
example 1
[0036] Example 1: a kind of preparation method of 4,6-dichloro-5-amino-2-propylthiopyrimidine, the steps are as follows:
[0037] Add 700g of 4,6-dihydroxy-2-mercaptopyrimidine to 10kg of water, add dropwise 5kg of 10% sodium hydroxide solution under stirring, control the temperature <25°C, add 5kg of methanol and 1.19kg of 1-bromopropane after the dropwise addition , stirred and reacted overnight at 40~45°C, cooled to room temperature, added dropwise dilute hydrochloric acid to adjust the pH to 2~3, stirred for 1h, filtered, washed with water, and dried under reduced pressure at 45°C to obtain 700g of 4,6-dihydroxy-2-propylsulfide base pyrimidine;
[0038] Add 910g of fuming nitric acid to 700g of glacial acetic acid, cool down to 0-5°C, add 700g of 4,6-dihydroxy-2-propylthiopyrimidine in batches, stir and react for 3 hours after adding, add water to crystallize, filter and wash with water , dried under reduced pressure at 45°C to obtain 728g of 4,6-dihydroxy-5-nitro-2-p...
example 2
[0042] Example 2: a kind of preparation method of 4,6-dichloro-5-amino-2-propylthiopyrimidine, the steps are as follows:
[0043] Add 1.4kg of 4,6-dihydroxy-2-mercaptopyrimidine to 20kg of water, add 10kg of 10% sodium hydroxide solution dropwise under stirring, control the temperature <25°C, add 10kg of methanol after the dropwise addition, 2.52kg of 1-bromo Propane, stirred and reacted overnight at 40~45°C, cooled to room temperature, added dropwise dilute hydrochloric acid to adjust the pH to 2~3, stirred for 1h, filtered, washed with water, and dried under reduced pressure at 45°C to obtain 1.42kg 4,6-dihydroxy-2- Propylthiopyrimidine;
[0044] Add 1.99kg of fuming nitric acid into 1.42kg of glacial acetic acid, cool down to 0-5°C, add 1.42kg of 4,6-dihydroxy-2-propylthiopyrimidine in batches, stir for 3 hours after the addition, add water to crystallize, Filter, wash with water, and dry under reduced pressure at 45°C to obtain 1.47kg of 4,6-dihydroxy-5-nitro-2-propyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com