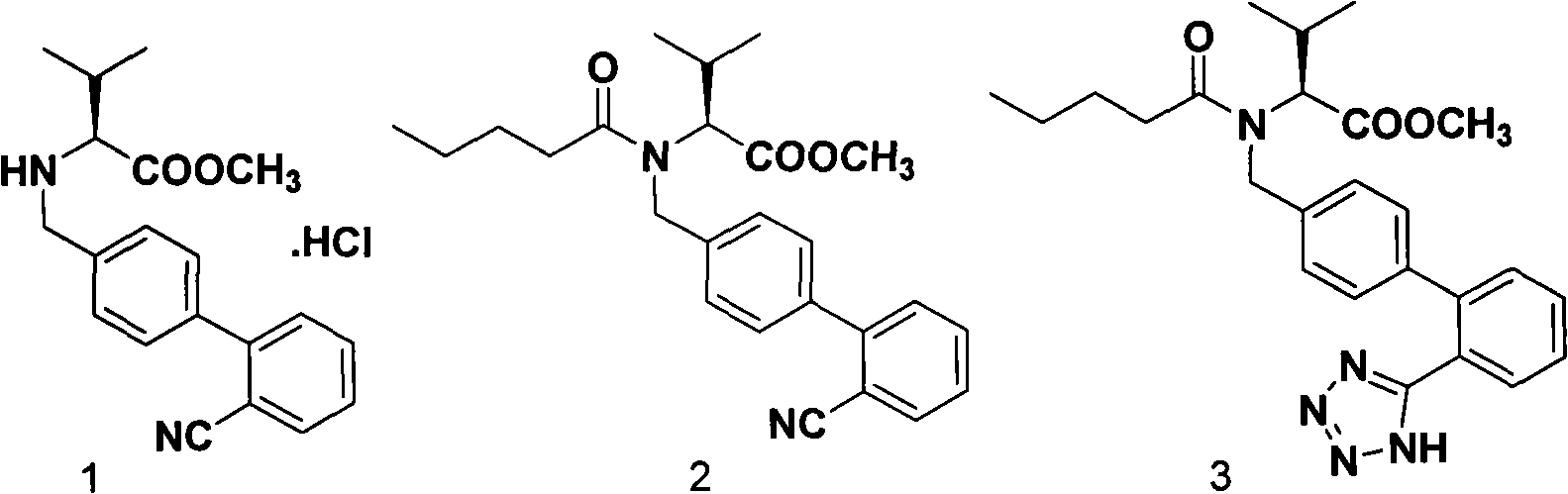
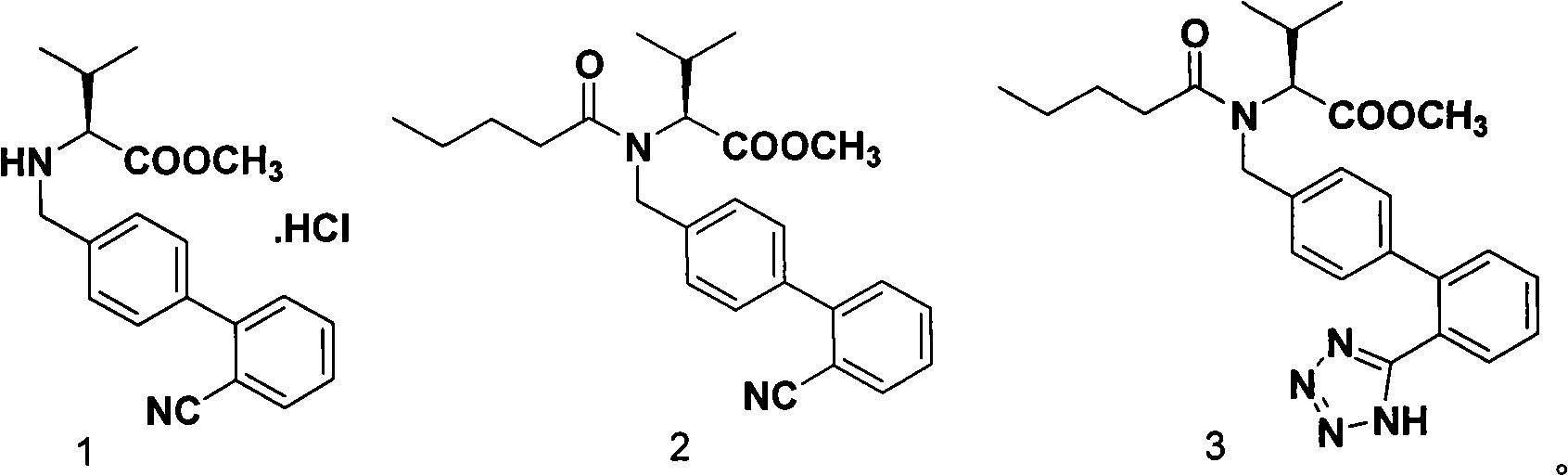
Improved process for synthesizing valsartan
A process and compound technology, applied in the field of drug synthesis, can solve problems such as difficult control, low yield, and difficult removal of heavy metal tin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the reaction kettle, add 1.4kg of compound 1, 14L of toluene, 1.58kg of triethylamine, stir, at 5°C, add 0.90kg of n-pentanoyl chloride, after the addition is complete, keep at 5-10°C and continue the reaction for 2 hours. After the reaction is complete, add 10kg water, stirring, standing to separate layers, and washing; the organic layer was dried and filtered to obtain a toluene solution of pentanoylated product 2;
[0023] In the toluene solution of the pentanoylated product 2, add Et 3 N·HCl 1.88kg and NaN 3 0.56kg, after reacting at 100-130°C for 28-32 hours, stop the reaction; after the reaction liquid is cooled to room temperature, filter, and distill under reduced pressure to remove toluene to obtain the cyclization product 3;
[0024] In the cyclization product 3, add 14L methanol, then add 3.69kg barium hydroxide octahydrate and 18L water, react at room temperature for 10-12h, after the reaction is finished, filter, decompress to remove methanol, the solut...
Embodiment 2
[0027] In the reactor, add 1.4kg of compound 1, 14L of toluene, 1.77kg of triethylamine, stir, at 10°C, add 1.18kg of n-pentanoyl chloride, after the addition is complete, keep at 10°C to continue the reaction for 2h, when the reaction is complete, add 10kg of water, Stir, let stand to separate layers, and wash; the organic layer is dried and filtered to obtain a toluene solution of pentanoylated product 2;
[0028] In the toluene solution of the pentanoylated product 2, add Et 3 N·HCl 3.22kg and NaN 3 0.89kg, after reacting at 90-120°C for 28-30 hours, stop the reaction. After the reaction solution was cooled to room temperature, it was filtered, and the toluene was distilled off under reduced pressure to obtain the cyclization product 3;
[0029] In the cyclization product 3, add 21L methanol, then add 5.54kg barium hydroxide octahydrate and 21L water, react at room temperature for 10-12h, after the reaction is completed, filter, distill methanol off under reduced pressure...
Embodiment 3
[0032] In the reaction kettle, add 1.4kg of compound 1, 7L of xylene, 1.38kg of triethylamine, stir at 10°C, add 0.94kg of n-pentanoyl chloride, after the addition is complete, keep at 10-15°C to continue the reaction for 2 hours. After the reaction is complete, add 10kg of water, stirred, allowed to stand to separate layers, and washed; the organic layer was dried and filtered to obtain a xylene solution of pentanoylated product 2;
[0033] In the xylene solution of the pentanoylated product 2, add Et 3 N·HCl 5.36kg and NaN 3 1.52kg, after reacting at 110-130°C for 28-32 hours, stop the reaction. After the reaction solution was cooled to room temperature, it was filtered, and the toluene was distilled off under reduced pressure to obtain the cyclization product 3;
[0034]To the cyclization product 3, add 7L of methanol, then add 1.67kg of barium hydroxide and 15L of water, react at room temperature for 10-12h, after the reaction, filter, distill off methanol under reduced ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com