Method for preparing epothilone D and B
A technology of epothilones and compounds, which is applied in the field of total synthesis of epothilones D and B, can solve the problems of low yield of epothilones, and achieve convenience for mass production, high yield, and few synthesis steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] In order to understand the present invention, the present invention is further illustrated below with examples, but it is not intended to limit the protection scope of the present invention.
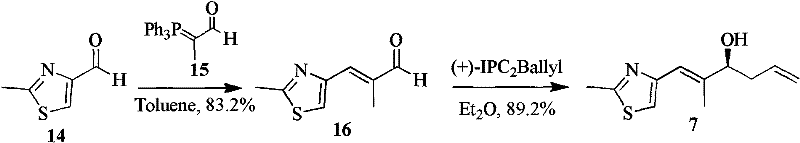
[0022] Synthesis of Epothilone D and B
[0023] Synthesis of (D)-Acetylcamphor-endesulphonamide (2)
[0024] Under the protection of nitrogen, add 4.1g sodium hydride (102.8mmol) into the round bottom flask, then add petroleum ether and stir for 30min, let stand, remove the supernatant, then add 500mL tetrahydrofuran into the round bottom flask and cool to 0°C, Add 20 g of camphor endosulphonamide (93.5 mmol) in 150 mL of tetrahydrofuran solution dropwise, stir for 1 h after the drop is complete, then add 8.3 mL of acetyl chloride (112.2 mmol) drop by drop, after the addition, the temperature rises to room temperature, stir overnight, after the reaction is complete Add 62mL of sodium bicarbonate aqueous solution (1.19M), stir for 20min, spin out most of THF, extract with ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com