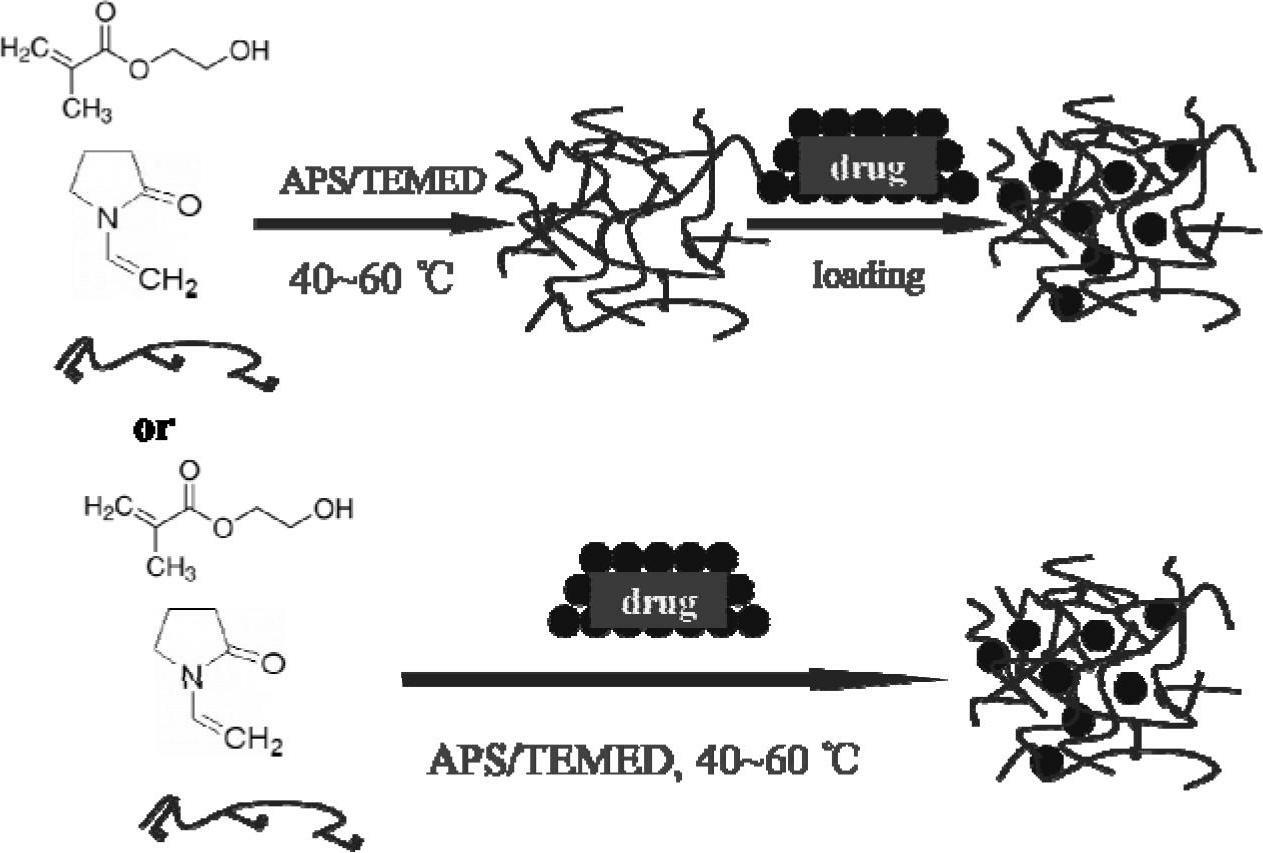
Hydrogel corneal contact lenses drug carrier
A hydrogel cornea and contact lens technology, which is applied in medical science, surgery, etc., can solve the problems of hydrogel elastic modulus drop, easy deformation, and inability to correct vision with corneal contact lenses, and achieve great social benefits and economic benefits. benefit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add hydroxyethyl methacrylate (HEMA) and N-vinylpyrrolidone (NVP) monomers to the container at a ratio of 7.4:2, and then add 1wt% hydroxyethyl methacrylate modified chitosan Acetic acid aqueous solution of sugar derivatives (CH, double bond graft rate 19%), wherein the quality of the chitosan derivatives is 0.4% of the total mass of HEMA and NVP (M 壳聚糖衍生物 :M HEMA+NVP ); Then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator that mol ratio is 1:1, wherein the quality of initiator is 0.5% (M of HEMA and NVP total mass APS+TMEDA :M HEMA+NVP ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体 系 ). Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel.
[0053] The gel time of this hydrogel is 25±2min; the transparency is good, and there is no obvious deformation after swelling; the equilibrium water...
Embodiment 2
[0055] Add hydroxyethyl methacrylate (HEMA) and N-vinylpyrrolidone (NVP) monomers to the container at a ratio of 7.4:2, and then add 1wt% hydroxyethyl methacrylate modified chitosan Acetic acid aqueous solution of sugar derivatives (CH, double bond graft rate 25%), wherein the quality of the chitosan derivatives is 0.3% of the total mass of HEMA and NVP (M 壳聚糖衍生物 :M HEMA+NVP ); Then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator that mol ratio is 1:1, wherein the quality of initiator is 0.5% (M of HEMA and NVP total mass APS+TMEDA :M HEMA+NVP ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体 系 ). Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel.
[0056] The gel time of this hydrogel is 25±2min; the transparency is good, and there is no obvious deformation after swelling; the equilibrium water...
Embodiment 3
[0058] Add hydroxyethyl methacrylate (HEMA) and N-vinylpyrrolidone (NVP) monomers to the container at a ratio of 7.4:2, and then add 2wt% hydroxyethyl methacrylate modified chitosan Acetic acid aqueous solution of sugar derivatives (CH, double bond graft rate 19%), wherein the quality of the chitosan derivatives is 0.4% of the total mass of HEMA and NVP (M 壳聚糖衍生物 :M HEMA+NVP ); Then add ammonium persulfate (APS), tetramethylethylenediamine (TMEDA) redox initiator that mol ratio is 1:1, wherein the quality of initiator is 0.5% (M of HEMA and NVP total mass APS+TMEDA :M HEMA+NVP ); the whole system adds or maintains the mass content of solvent water to 35% (M Water :M 总体 系 ).
[0059] Stir the reaction system evenly and pour it into a mold, react at 60° C. for 1 hour, then soak in water for demoulding to obtain a terpolymer hydrogel. The gel time of this hydrogel is 25±2min; the transparency is good, and there is no obvious deformation after swelling; the equilibrium water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com