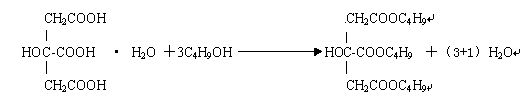Preparation process for plasticizer-tributyl citrate
A plasticizer citric acid, production process technology, applied in the direction of carboxylic acid ester preparation, organic compound preparation, chemical recovery, etc., can solve the problem that no recovery and recycling method has been found, large amount of catalyst used, serious corrosion of equipment, etc., Achieve the effect of reducing material consumption, short reaction time and reducing pollution discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
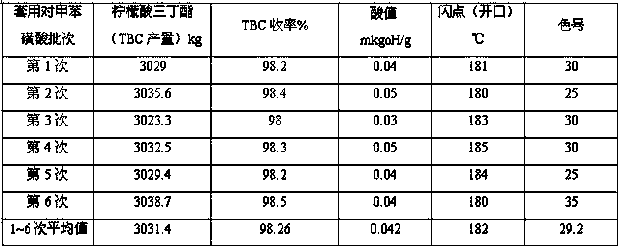
[0025] In the 5 m3 enamel kettle equipped with three-layer paddle agitator, temperature display instrument, esterification tower, condenser, and water separator, 1600kg of butanol was dropped into for the first time (all recovered alcohol in the previous batch was added after 100% discount, and then added Add fresh butanol to 1600kg, accounting for 70% of the total feeding amount), start stirring, add p-toluenesulfonic acid 25kg, activated carbon 8kg, quickly add citric acid monohydrate 1450kg, pass N 2 (N 2 , about 2m3 per hour). Steam through the jacket to make the temperature of the material rise steadily to 125-130°C, slowly add 700kg of fresh butanol for the second time, control the esterification temperature at 125-135°C, the pressure of the kettle is ≤0.015Mpa, and measure the acid value every 30min. When the acid value is ≤2.3mgKOH / g, it is the end point of esterification, and the reaction time is about 7-8 hours. Carry out the first dealcoholization in the esterific...
Embodiment 2
[0027] Except the input amount adjustment of each raw material, all the other reaction parameters are identical with embodiment 1, and detailed experimental data are as follows: .
Embodiment 3
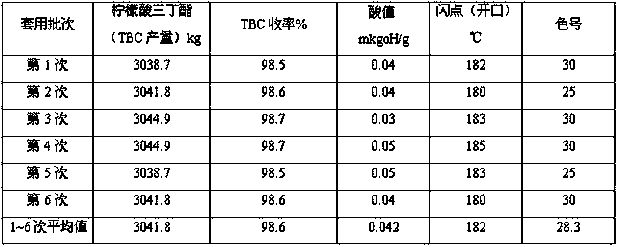
[0029] Except the input amount adjustment of each raw material, all the other reaction parameters are identical with embodiment 1, and detailed experimental data are as follows:
[0030] .
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com