Amine-epoxy adducts and their use for preparing polyurea and polyurea-polyurethane coatings
A technology of epoxy adducts and adducts, applied in the field of amine-epoxy adducts, amine-epoxy adducts and adduct blends, to achieve good properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Mixtures of the amines listed above can also be used for easier adjustment of the reaction time with the isocyanate during the preparation of the coating. For example, it is preferred to use amine conjugates in which at least 50% of the amino groups in the resulting adduct are secondary amino groups.
[0042] For the preparation of slowly reacting adducts with isocyanates, some aliphatic, cycloaliphatic, araliphatic and aromatic primary monoamines are particularly preferred due to their steric hindrance, for example: 2-Ethylaniline, 2- Methylcyclohexylamine, tert-octylamine and similar amines. Secondary amines of the bis-aspartate class (for example, the Desmophen product from Bayer) are also preferred for adjusting the isocyanate reactivity due to their various structures.
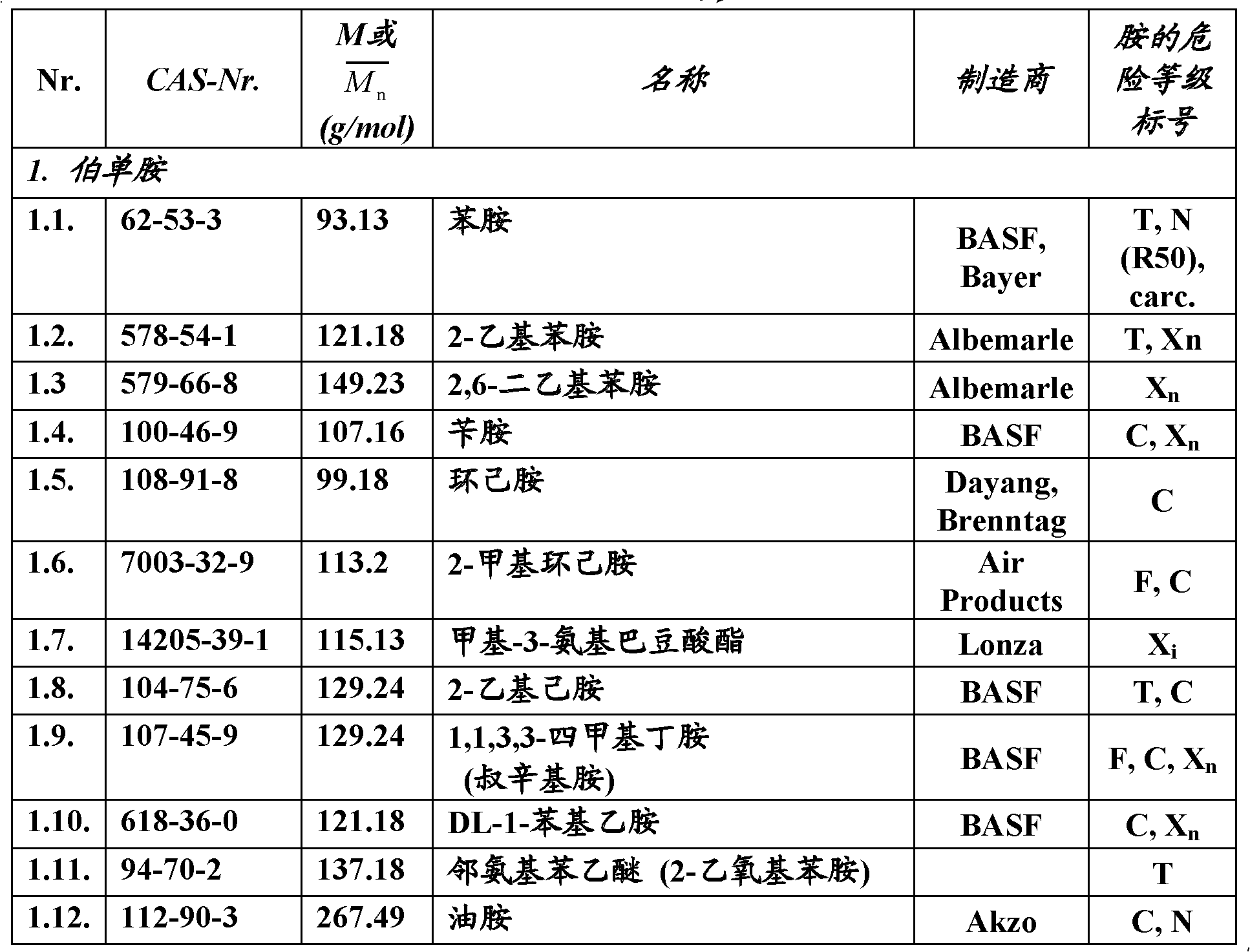
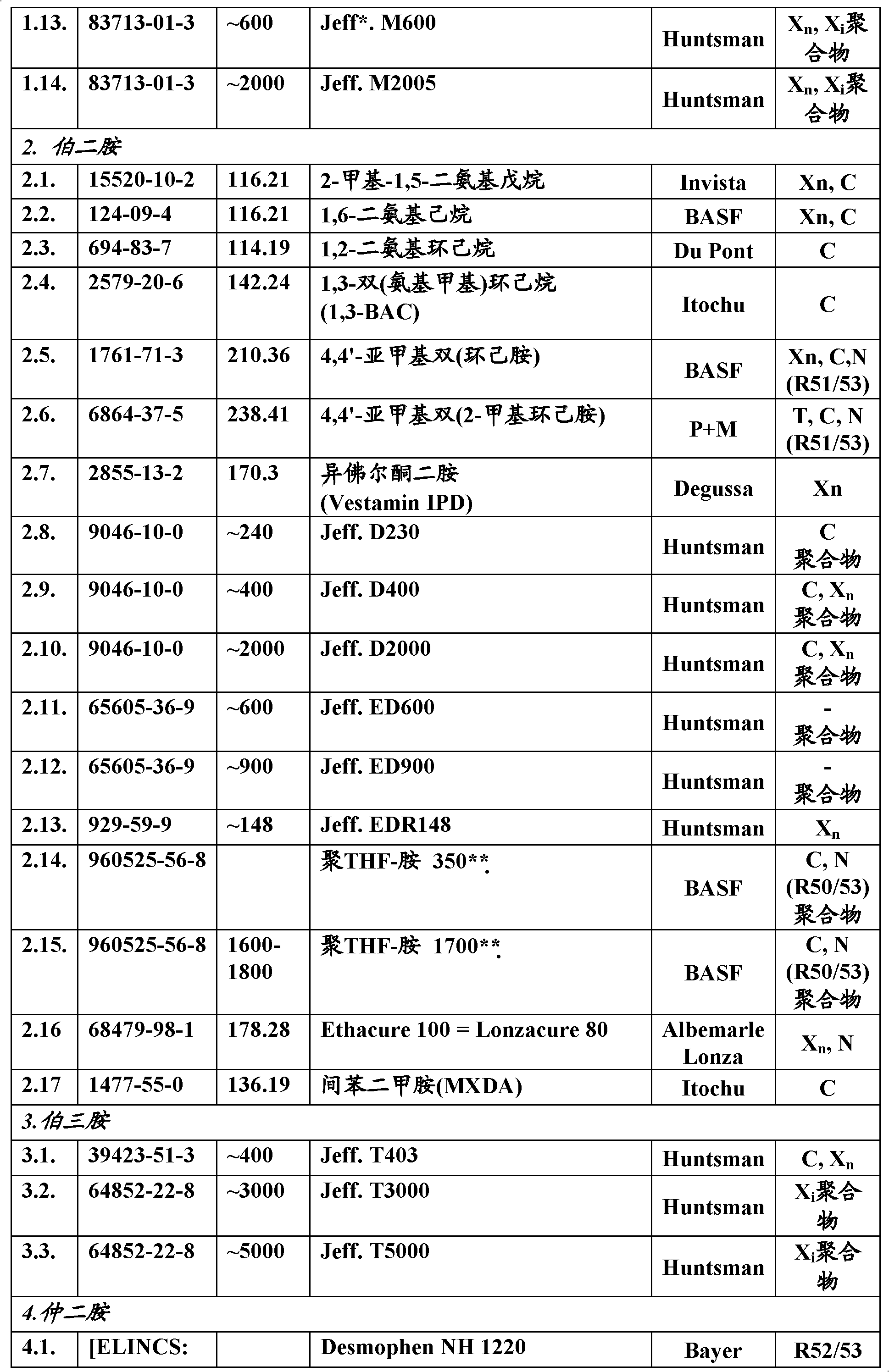
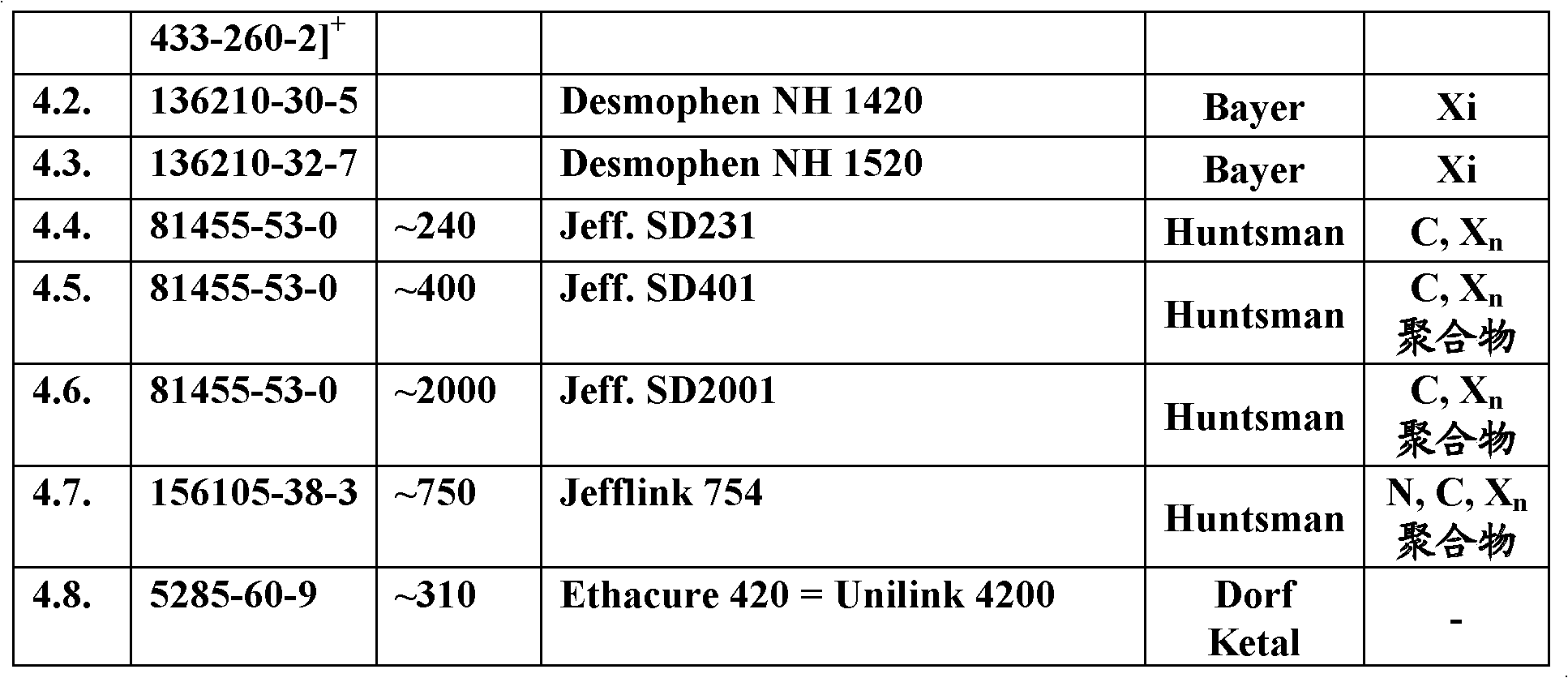
[0043] For the adducts according to the invention, the epoxy compounds listed in Table 2 are preferred as epoxy components. Parameters of the starting epoxy components Table 2.
[0044]
[00...
Embodiment 1
[0095] Embodiment 1 (contrast)
[0096] Laboratory-scale preparation of the adduct "MA-0", Nr.1.
[0097] Starting components:
[0098] 1mol 1,5-diamino-2-methylpentane (MPMD)+1mol AH-17
[0099] In a 700ml conventional open metal can with a top injection hole of 80mm diameter used in the paint industry, 116g MPMD and 214g AH-17 were weighed. Under continuous stirring, it was heated to 60±2° C. by means of an electric basket heater. At this temperature a conversion of 81% was reached within 1.5 hours, then, after a further two hours at 90±1° C. the conversion had reached 98.5%. The reactivity and consumption of epoxy groups was monitored by determining the amount of amine and epoxy. The average viscosity of all adducts prepared was 350±20 mPa.s at 20°C, 44±10 mPa.s at 50°C and 17±5 mPa.s at 70°C.
Embodiment 2
[0103] Laboratory-scale preparation of the adduct "PA112", Nr.7
[0104] Starting components:
[0105] 1mol Jeffamine D230+2mol AH-17
[0106] Weigh 120g of Jeffamine D230 aliphatic polyetherdiamine into a 500ml four-neck vulcanization flask equipped with a dropping funnel and a thermometer, and heat it to 70±2.5°C with a basket electric heater under vigorous and continuous stirring. While maintaining this material, and subsequently the reaction mixture, at this temperature for two hours, 222 g of 2-ethylhexyl glycidyl ether were slowly added to the flask over 2 hours under continuous and vigorous stirring. Depending on the epoxy content in the reaction mixture, it was kept at the specified 70±2.5°C for an additional 1.5 hours and at 90±25°C for an additional 1.5 hours. The reactivity and consumption of epoxy groups was monitored by determining the amount of amine and epoxy groups, and after complete consumption of the starting epoxy groups, the reaction mixture was rapidly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Adhesive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com