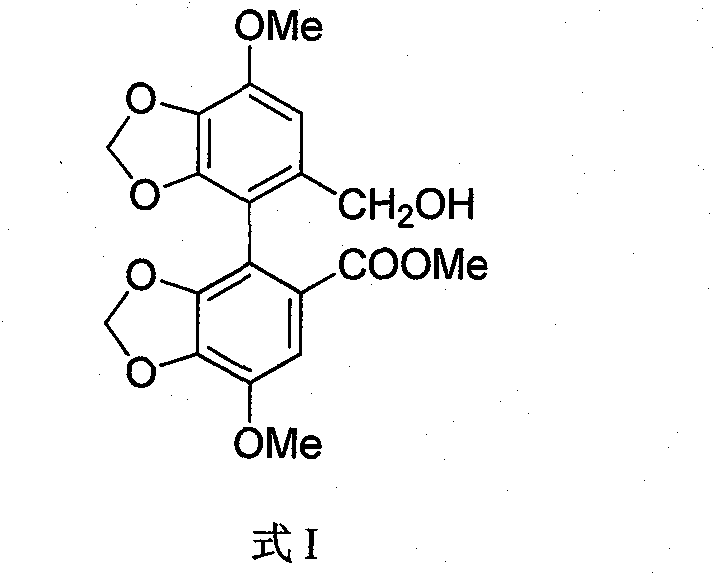
Novel method for preparing bicyclol
A bicyclic alcohol and methanol technology, applied in the field of bicyclic alcohol preparation, can solve the problems of long reaction steps, cumbersome operation, and low total yield, and achieve the effects of mild reaction conditions, simple operation, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Synthesis of 1-(3,5-dinitro)-benzoyl-4-methylpiperazine (X)
[0046] 3,5-Dinitrobenzoic acid (4.24g, 20mmol) was dissolved in thionyl chloride (8.7mL, 120mmol), refluxed at 90°C for 4 hours, a small amount of toluene was added, and the solvent was evaporated to obtain a light yellow solid. The solid was dissolved in 50 mL of chloroform, and added dropwise to another reaction bottle (containing 50 mL of chloroform, 2.8 g of potassium carbonate, and 2.7 mL of N-methylpiperazine) under ice-bath conditions, and the reaction was completed after 4 hours. Wash with water (4×100mL), dry the organic phase by adding anhydrous potassium carbonate, filter, concentrate, and recrystallize from chloroform-cyclohexane to obtain 4.12g of light yellow needle-like crystals, keep away from light, melting point 135-137°C, two steps The overall reaction yield was 70.1%.
[0047] 1 H-NMR (500MHz, d 6 -DMSO) δ: 8.87(t, J=2.0Hz, 1H), 8.56(d, J=2.0Hz, 2H), 3.54(s, 4H), 2.42(t, J...
Embodiment 2
[0048] Embodiment 2: Synthesis of 3,4-dihydroxy-5-methoxybenzoic acid methyl ester (VIII)
[0049]Take methyl gallate (30.00g, 163.04mmol) and borax 36g and dissolve in 280mL water, stir for 1h to dissolve completely, under ice bath condition, slowly add dropwise 12g sodium hydroxide (dissolved in 50mL water) and dimethyl sulfate (21.6 mL, 228.26mmol), reacted at room temperature for 9h, detected by TLC (DCM:MeOH=12:1), the reaction was complete, the pH of the reaction solution was adjusted to about 8, extracted with chloroform (2×50mL), the organic phase was removed, and the aqueous phase was treated with concentrated sulfuric acid Adjust the pH to about 3, extract with ethyl acetate, dry the organic phase with anhydrous sodium sulfate, filter, concentrate, and recrystallize from absolute ethanol to obtain a white solid, filter, and dry to obtain 25.33 g of a white solid with a melting point of 112-115 ° C. The rate is 78.2%.
Embodiment 3
[0050] Embodiment 3: Synthesis of 2-bromo-3,4-dihydroxy-5-methoxybenzoic acid methyl ester (IX)
[0051] Methyl 3,4-dihydroxy-5-methoxybenzoate (1.58g, 8mmol) was dissolved in 45mL of chloroform, stirred, and dibromohydantoin (1.42g, 4.08mmol) was slowly added in batches to react at room temperature for 20h, detected by TLC (DCM: MeOH=70:1) the reaction is complete, add 10% Na 2 S 2 o 4 The solution was washed (2×20mL), the organic phase was dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated to dryness, and a yellowish-brown solid was precipitated, which was washed with a small amount of ether to obtain 2.07g of a white solid with a melting point of 140-141°C and a yield of 93.1% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com