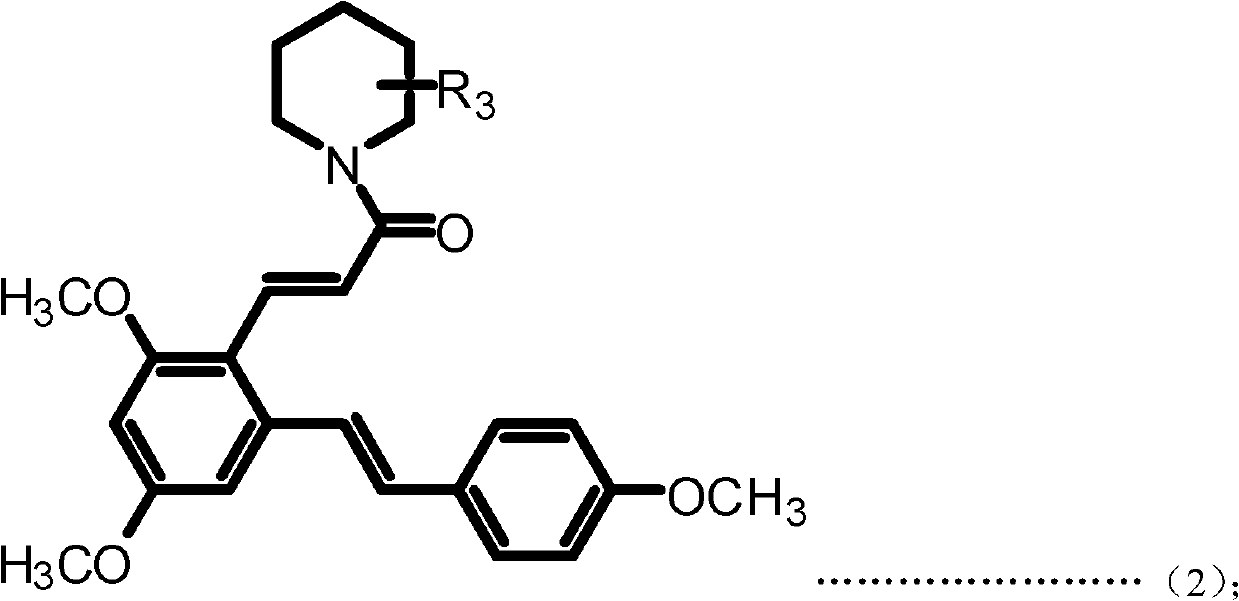
Resveratrol benzene acrylamide derivative, preparing method and application thereof
A technology of alcohol phenylacrylamide and phenylacrylamide, applied in the field of resveratrol derivatives, can solve the problems that resveratrol cannot be used as an anticancer drug, photosensitivity and metabolic instability of resveratrol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
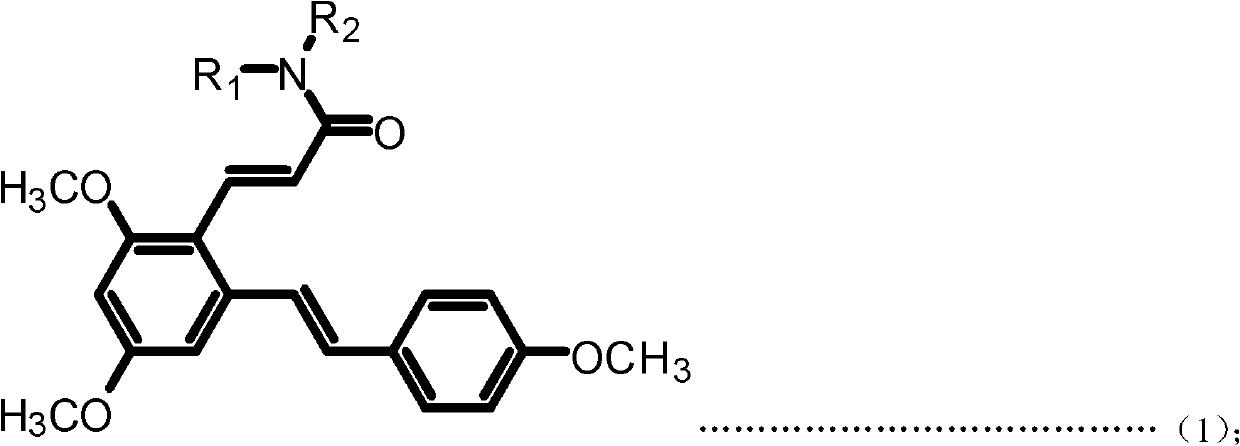
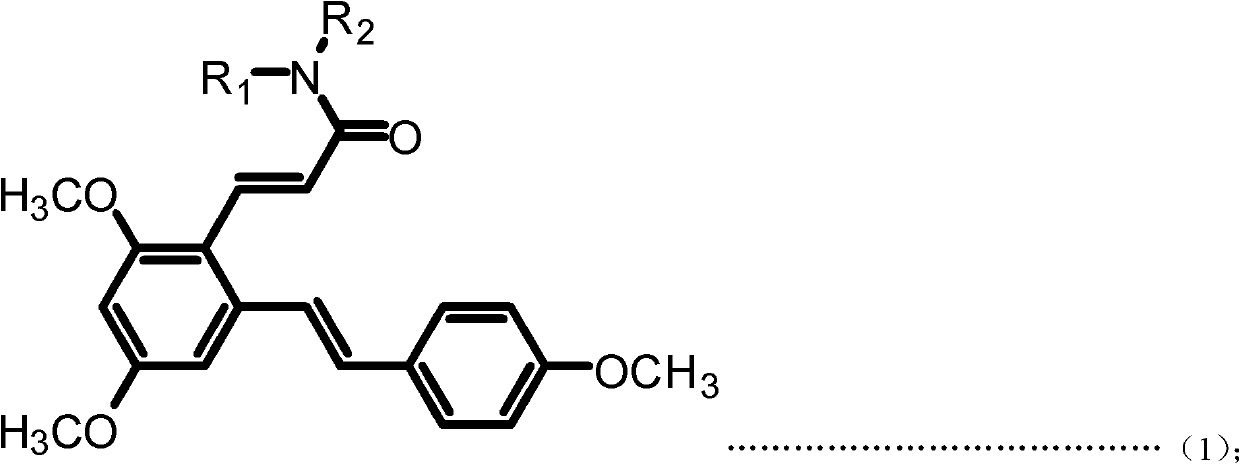
[0048] Example 1: (E)-3-(2,4-dimethoxy-6-((E)-4-methoxystyrene)phenyl)-N-ethylacrylamide (Compound 1) preparation
[0049]
[0050] a. Add resveratrol 228mg (1mmol) and 10mL acetonitrile to the 50mL single-neck bottle, add DMF dropwise under magnetic stirring until the reaction solution changes from milky white turbidity to light yellow clear solution; then dropwise add phosphorus oxychloride 230mg under an ice-water bath (15mmol), stirred at room temperature for 30min after the dropwise addition; then reacted at 50°C for 3h, after the reaction was completed, filtered, dried, and subjected to column chromatography (ethyl acetate:petroleum ether=1:1 (volume)) to obtain ( E)-2,4-Dihydroxy-6-(4-hydroxystyrene)benzaldehyde (Intermediate I). Yellow solid, 95.7% yield. m.p.210-212℃; 1 H NMR (DMSO-d6): δ (ppm) 6.21 (s, 1H), 6.62 (s, 1H), 6.78 (d, 2H, J=8.4Hz), 7.02 (d, 1H, J=16.0Hz), 7.49(d, 2H, J=8.4Hz), 7.70(d, 1H, J=16.2Hz), 9.71(s, 1H), 10.27(s, 1H), 10.76(s, 1H), 12.12(s,...
Embodiment 2
[0054] Example 2: (E)-3-(2,4-dimethoxy-6-((E)-4-methoxystyrene)phenyl)-N-propylacrylamide (compound 2) preparation
[0055]
[0056] The preparation method is the same as in Example 1, except that in step d, propylamine is used instead of ethylamine to obtain the target product (E)-3-(2,4-dimethoxy-6-((E)-4-methoxyl group) Styrene)phenyl)-N-propylacrylamide. White solid, 83.1% yield, m.p.142-145°C; 1 H NMR (300MHz, CDCl 3 ): δ(ppm) 0.95(t, 3H, J=7.5Hz), 1.54-1.63(m, 2H), 3.33(q, 2H, J=6.6Hz), 3.83(s, 3H), 3.85(s, 3H), 3.87 (s, 3H), 5.55 (bras, 1H), 6.33 (d, 1H, J=15.6Hz), 6.40 (d, 1H, J=2.1Hz), 6.70 (d, 1H, J=2.1 Hz), 6.88-6.93(m, 3H), 7.28(d, 1H, J=15.9Hz), 7.44-7.46(m, 2H), 7.94(d, 1H, J=15.6Hz). MS(ESI): 382.5 (C 23 H 27 NO 4 , [M+H] + ).Anal.Calcd for C 23 H 27 NO 4 : C, 72.42; H, 7.13; N, 3.67%; Found: C, 72.30; H, 7.15; N, 3.68.
Embodiment 3
[0057] Example 3: Preparation of (E)-N-butyl-3-(2,4-dimethoxy-6-((E)-4-methoxystyrene))benzeneacrylamide (Compound 3)
[0058]
[0059] The preparation method is the same as in Example 1, except that in step d, butylamine is used instead of ethylamine to obtain the target product (E)-N-butyl-3-(2,4-dimethoxy-6-((E) -4-Methoxystyrene)) phenylacrylamide. White solid, 87.2% yield, m.p.153-155°C; 1 HNMR (300MHz, CDCl 3 ): δ(ppm) 0.93(t, 3H, J=7.2Hz), 1.34-1.41(m, 2H), 1.49-1.56(m, 2H), 3.34-3.40(m, 2H), 3.83(s, 3H) ), 3.85(s, 3H), 3.88(s, 3H), 5.49(bras, 1H), 6.32(d, 1H, J=15.6Hz), 6.41(d, 1H, J=2.4Hz), 6.70(d , 1H, J=2.4Hz), 6.88-6.93 (m, 3H), 7.28 (d, 1H, J=16.2Hz), 7.43-7.46 (m, 2H), 7.93 (d, 1H, J=15.6Hz) .MS(ESI): 396.5(C 24 H 29 NO 4 , [M+H] + ).Anal.Calcdfor C 24 H 29 NO 4 : C, 72.89; H, 7.39; N, 3.54%; Found: C, 72.73; H, 7.41; N, 3.55.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com