Method for synthesizing tebuthiuron technical material
A technology for the synthesis of terbuthiazide and its synthetic method, applied in the direction of organic chemistry, can solve the problems of low content, low yield, difficult separation and purification, etc., and achieve the effects of high product yield, excellent product quality, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
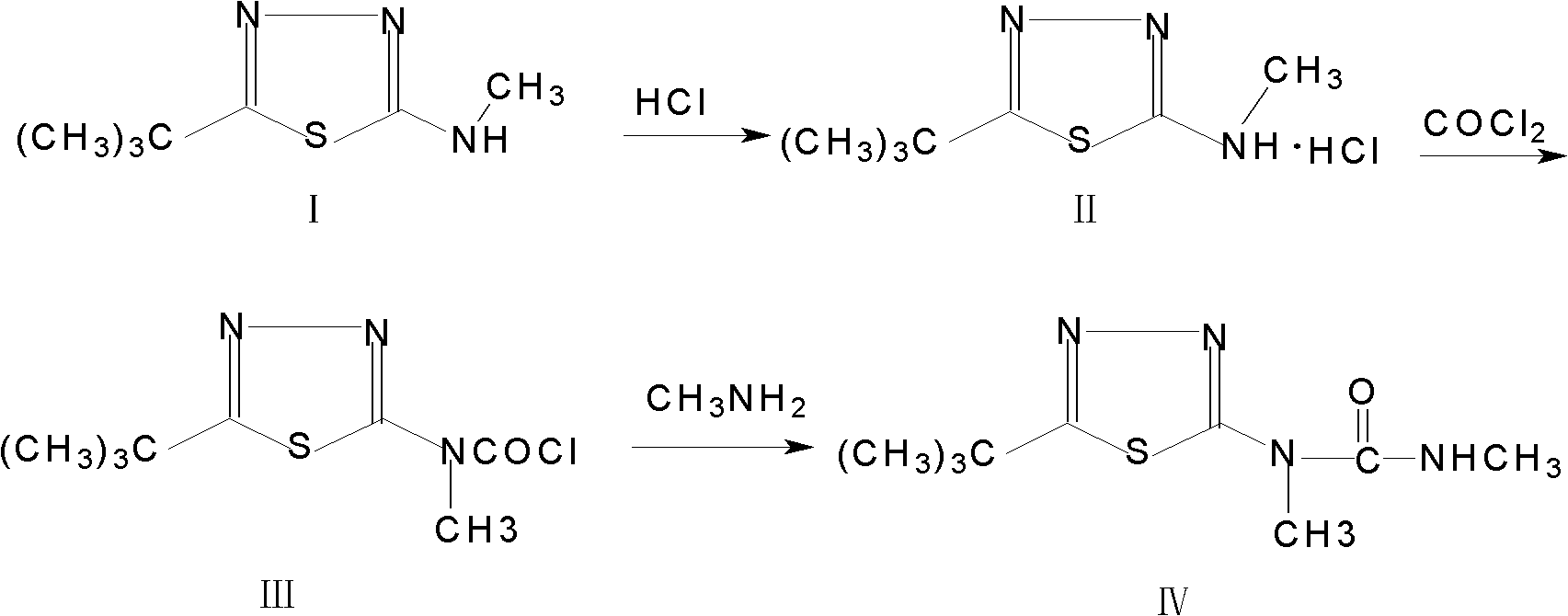
[0025] (1) Put 500 ml of toluene and 51.3 g (0.3 mol) of 5-tert-butyl-2-methylamino-1,3,4-thiadiazole (I) into a 1000 ml four-necked flask. While stirring, 46 g of 31% industrial hydrochloric acid was added dropwise. After the drop, the temperature was raised to reflux to azeotropically remove the water in the system to obtain 5-tert-butyl-2-methylamino-1,3,4-thiadiazole hydrochloride (II).
[0026] (2) Cool the toluene suspension of 5-tert-butyl-2-methylamino-1,3,4-thiadiazole hydrochloride (II) prepared above to 85° C. Pass through phosgene at a speed of min, and after passing light for 2 hours, the material becomes clear and transparent, and stop passing through phosgene. Introduce nitrogen to drive away residual phosgene and hydrogen chloride gas to obtain N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamoyl chloride (III) toluene solution.
[0027] (3) Cool down the toluene solution of N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamoyl chloride (III) prepared above ...
Embodiment 2
[0030] (1) drop into 1000L of toluene in the enamel kettle of 2000 liters, 5-tert-butyl-2-methylamino-1,3,150kg of 4-thiadiazole (I), under stirring state, drop 31% industrial Hydrochloric acid 124kg. After the drop, the temperature was raised to reflux, and the water in the system was azeotropically removed to obtain 5-tert-butyl-2-methylamino-1,3,4-thiadiazole hydrochloride (II).
[0031] (2) Cool the toluene suspension of 5-tert-butyl-2-methylamino-1,3,4-thiadiazole hydrochloride (II) prepared above to 85° C. 3 The flow rate of / h was passed through phosgene, and after 4 hours of light reaction, the material became clear and transparent, and the passage of phosgene was stopped. Introduce nitrogen to drive away residual phosgene and hydrogen chloride gas. N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamoyl chloride (III) toluene solution was obtained.
[0032] (3) Cool the toluene solution of N-methyl-N-(5-tert-butyl-1,3,4-thiadiazole) carbamoyl chloride (III) prepared ...
Embodiment 3
[0035] (1) Put 500 ml of toluene and 51.3 g (0.3 mol) of 5-tert-butyl-2-methylamino-1,3,4-thiadiazole (I) into a 1000 ml four-necked flask. Under stirring, hydrogen chloride gas was introduced to saturation to obtain 5-tert-butyl-2-methylamino-1,3,4-thiadiazole hydrochloride (II).
[0036] Light transmission, amination, and water washing are the same as steps (2), (3) and (4) of Example 1. The obtained terbuthiauron technical product was 68.0 g, the content was 98.2%, and the yield was 97.6% (calculated as 5-tert-butyl-2-methylamino-1,3,4-thiadiazole).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com