Method of treatment of liver disease
A liver disease, calcium channel blocker technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, plant raw materials, etc., can solve problems such as the unconfirmed effect of calcium blockers on hepatic artery blood flow, etc., to reduce silymarin Effects of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0099] Despite the complex composition of silymarin, other researchers have shown that this botanical agent can be formulated in erodible matrices using systems based on glyceryl monostearate and polyethylene glycol 6000 or poloxamer 188 (Cheng et al. 2007). Silymarin and other botanical antioxidants can thus be co-formulated with diltiazem using a number of standard techniques for preparing sustained release formulations. The claims of the present invention relate to the concept of co-formulation of botanical antioxidants with low doses of diltiazem in sustained release formulations, rather than the specific chemical nature of the formulations used.
Embodiment 1
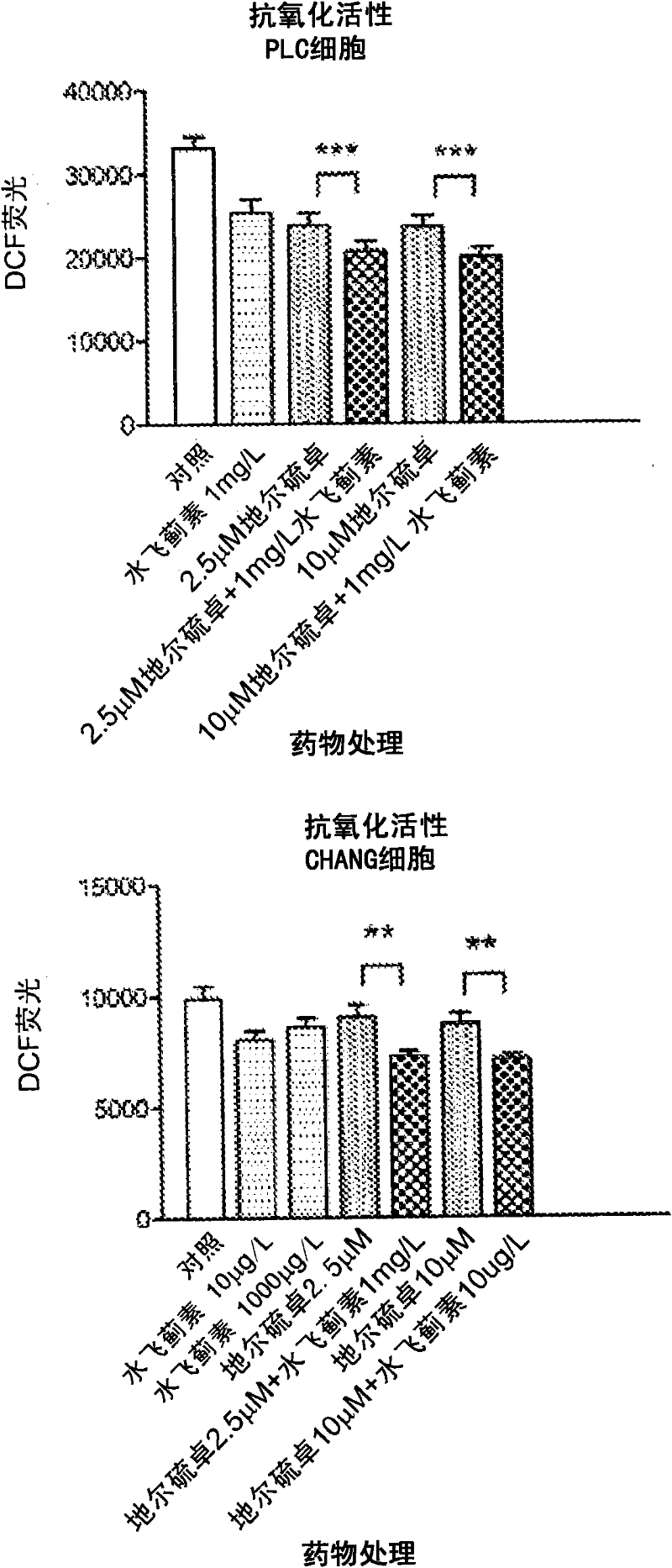
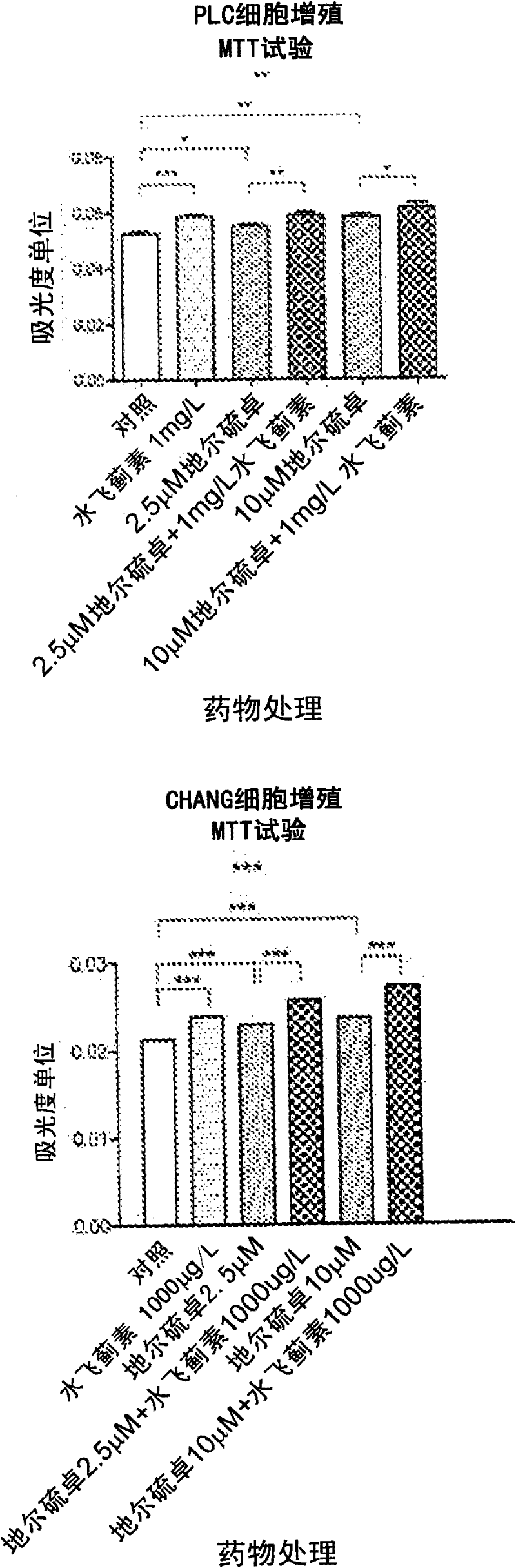
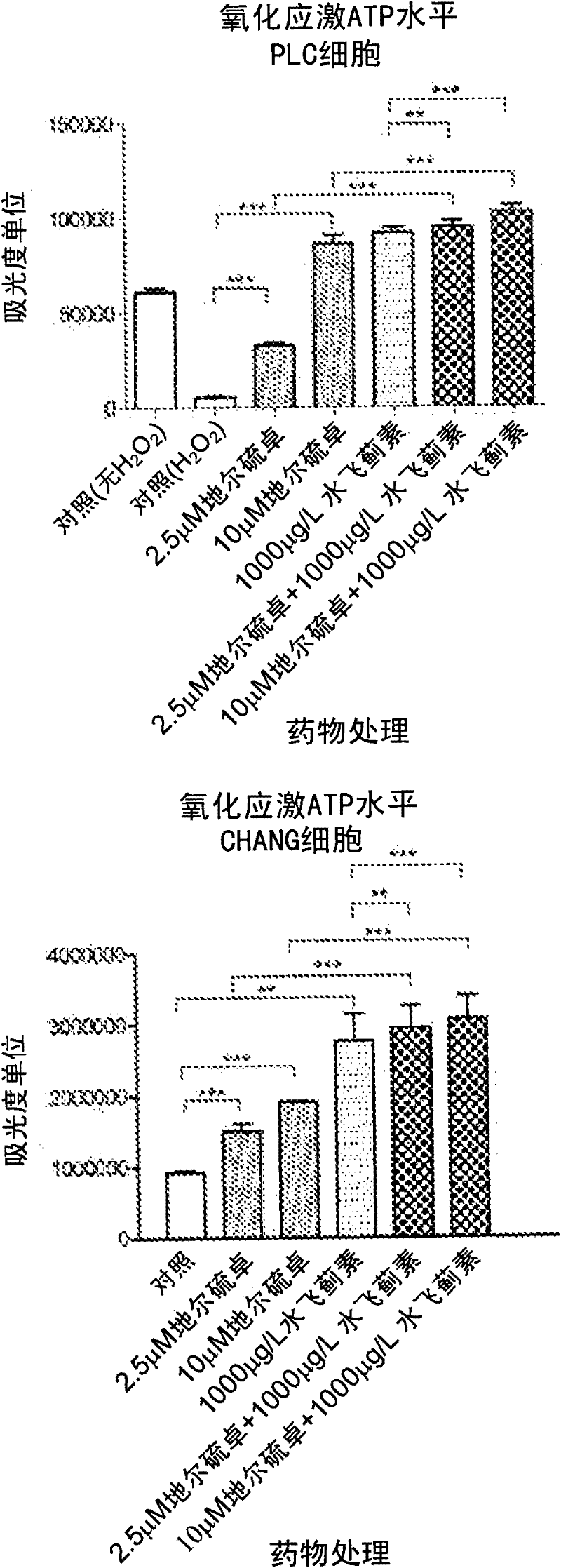
[0109] This example compares the response of hepatocytes to diltiazem, silymarin and the combination of diltiazem and silymarin under oxidative stress.
[0110] Materials and methods
[0111] Including d-cis-diltiazem, silymarin, and dichlorofluoroacetoacetate (DCFH 2 -DA) were purchased from Sigma Chemical, St. Louis, MO. Darwin's modified Elder's medium (DMEM), fetal bovine serum, penicillin and streptomycin were purchased from GIBCO / BRL. MTT (cell proliferation) and ATPase kits were purchased from Sigma (St. Louis, MO). The human hepatoma cell line Chang and PLC / PRF / 5 cells were purchased from the American Type Culture Collection, Rockville, MD (ATCC, Rockville, MD).
[0112] Cell Culture and Drug Therapy
[0113] Chang and PLC / PRF / 5 hepatoma cells in 25 cm supplemented with DMEM 2 in culture flasks at 37°C in 95% air and 5% CO 2 Maintain at least 24 hours in a humidified atmosphere in DMEM containing 10% FBS (DF-10), penicillin and streptomycin (50 units / ml). Cells ...
Embodiment 2
[0148] Example 2: Metabolism studies of diltiazem and silymarin
[0149] The procedure of this example was performed with the rat hepatic cell line H4-IIE cell line. This is a well established hepatocyte model. The cell line exhibits all the main features of normally differentiated rat hepatocytes. This cell line has been used for medical and biochemical research on liver function for 20 years, and its characteristics are not inferior to other normal freshly isolated rat hepatocytes. Oxidative damage was induced by an established method using hydrogen peroxide. Hepatocytes cultured in plastic multiwell plates were incubated with 0.5 mM hydrogen peroxide for 1 hour and then incubated in normal medium for 12 hours under standard incubation conditions. This regimen induces oxidative damage to target cells. Cell damage was assessed by measuring the exclusion of the dye trypan blue and the release of the cytosolic enzyme lactate dehydrogenase.
[0150] To evaluate the rate o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com