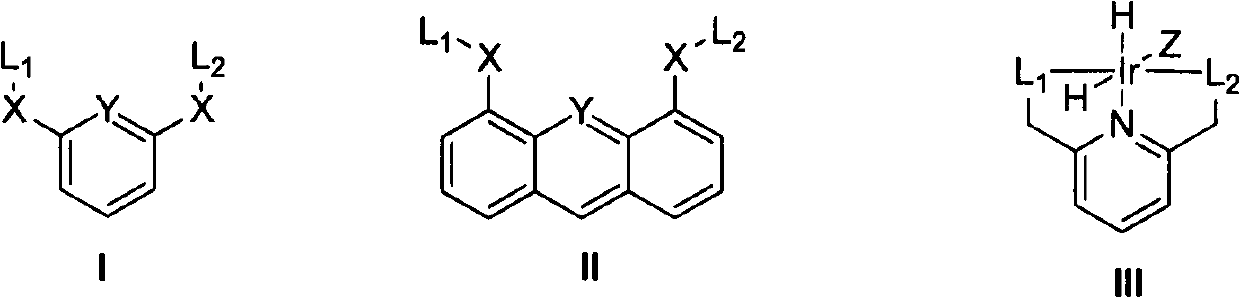
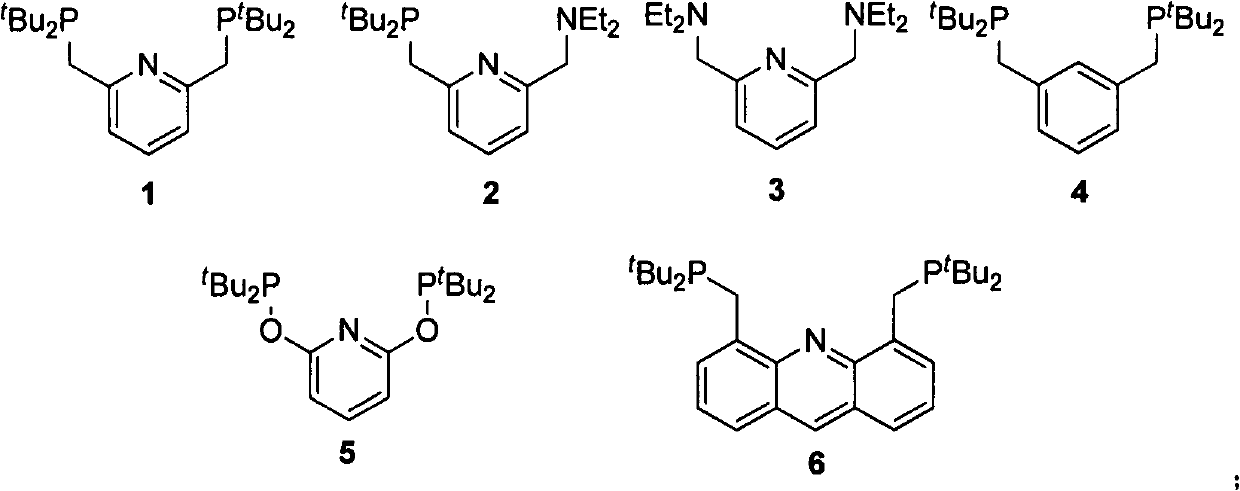
Process for preparing gamma-valerolactone by utilizing iridium-pincer ligand complex catalyst
A technology of pincer ligands and complexes, which is applied in the field of preparation of γ-valerolactone, can solve problems such as difficult large-scale production, large amount of catalyst used, harsh reaction conditions, etc., and achieves mild reaction conditions, high yield, and reaction good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
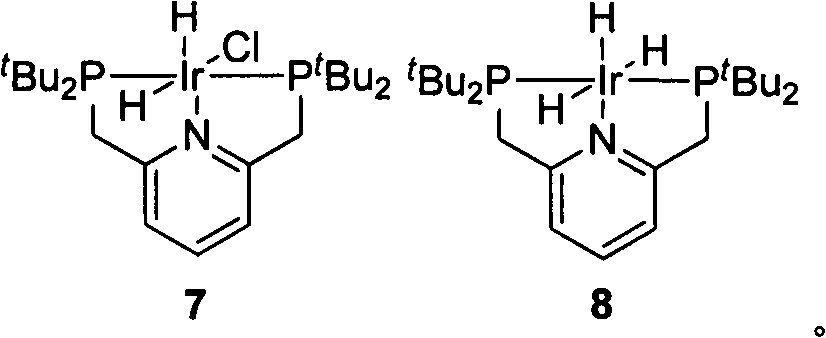
[0030] Preparation of Catalyst 7
[0031]
[0032] Add to a dry and clean 10mL Schlenk tube t Bu-PNp (Compound 1) (119mg, 302μmol) and [Ir(coe) 2 Cl] 2 (90mg, 100μmol), replace the system with an argon atmosphere, add degassed tetrahydrofuran (5.5mL), stir and dissolve evenly, transfer the solution to an argon-protected high-pressure hydrogenation kettle, replace the argon three times quickly, and replace the hydrogen After five times, the hydrogen pressure was adjusted to 2.5 MPa, the oil bath was heated to 90°C, and the reaction was stirred for 12 hours. Cool to room temperature, transfer the system to another clean and dry 10mL Schlenk tube under the protection of argon, desolvate in vacuum to a residual volume of about 0.5mL, add degassed n-hexane (5mL), and precipitate a white precipitate. It was filtered under protection, and the filter cake was washed with degassed n-hexane (2×5 mL) and dried in vacuo to obtain the product 7 (100 mg, 80%) as a white solid. 1 H NM...
Embodiment 2
[0034] Preparation of Catalyst 8
[0035]
[0036] Add ( t Bu-PNP)IrH 2 Cl(7) (125mg, 200μmol), sodium hydride (500mg, 20mmol), after replacing the system with argon atmosphere, add degassed tetrahydrofuran (6mL), heat the oil bath to 50°C and stir for 16 hours, then cool to room temperature After filtering under the protection of argon, the filtrate was transferred to another dry and clean 10mL Schlenk tube, and the volume of the system was vacuum precipitated to about 0.5mL, and degassed n-hexane (5mL) was added, and a brown solid was precipitated. The mixture was filtered under the hood, and the filter cake was washed with degassed n-hexane (2×5 mL), and dried in vacuo to obtain khaki solid product 8 (50 mg, 42%). 1 H NMR (400MHz, C 6 D. 6 )δ6.82(t, J=7.7Hz, 1H), 6.50(d, J=7.7Hz, 2H), 3.13(s, 4H), 1.42(t, J=6.4Hz, 36H), -10.12(td , J=15.3, 5.2Hz, 2H), -19.78(tt, J=14.8, 4.8Hz, 1H). 31 P NMR (162MHz, C 6 D. 6 )δ88.03(s). 1 H NMR (400MHz, CD 2 Cl 2 )δ7.36(t, J=7.6...
Embodiment 3~8
[0038] Weigh [Ir(coe) in the glove box 2 Cl] 2 (1.3mg, 1.5μmol) and Bu-PNP (compound 1) (1.8mg, 4.5μmol) in a dry and clean 10mL Schlenk tube, replace the system with argon atmosphere and add the corresponding degassed solvent (2mL), The water bath was heated to 50° C., stirred and complexed for 30 minutes, and then replaced with a hydrogen atmosphere (hydrogen balloon) to continue stirring and complexed for 15 minutes. A solution of levulinic acid (348.3mg, 3mmol) prepared in advance dissolved in the corresponding solvent (2mL) was added to the above catalyst solution, after stirring evenly, the system was added to a weighing machine replaced by an argon atmosphere with 1.2 equivalents of hydroxide In the high-pressure hydrogenation kettle of potassium (85% purity) (230mg, 3.5mmol), after rapidly displacing the argon three times, rapidly displace the hydrogen five times, adjust the hydrogen pressure to 5Mpa, heat the oil bath to 100°C, stir the reaction (1250rpm) for 15 Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com