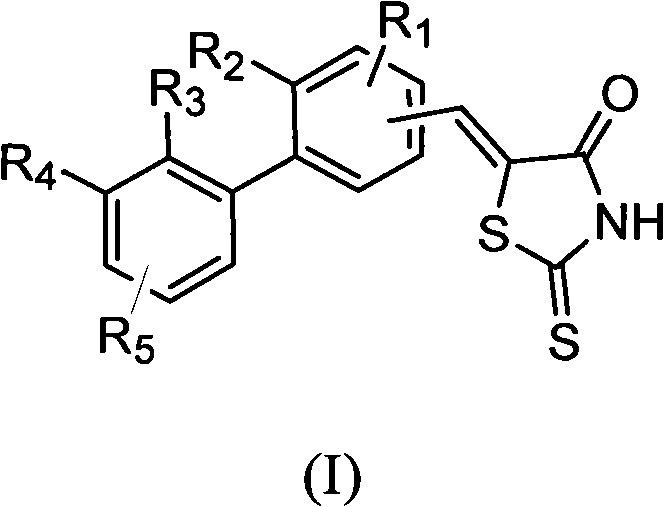
Biphenyl methylene-2-sulpho-4-thiazolone compound as well as preparation method and application thereof
A technology of biphenylmethylene and benzylidene, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of impaired insulin signaling in insulin resistance and increased catalytic activity of PTP1B dephosphorylation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
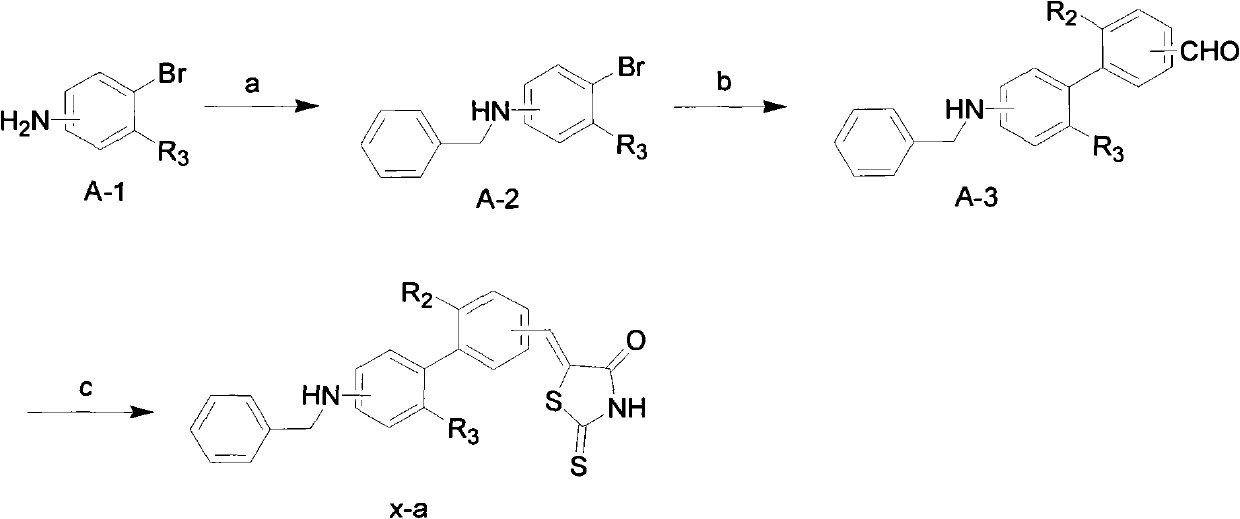
[0066] Example 1: 5-[3-(3-benzylaminophenyl)benzylidene]-2-thio-4-thiazolone (compound 1-a)
[0067]
[0068] Referring to the preparation process of the aforementioned compound x-a, compound 1-a was prepared:
[0069] a) Dissolve 375mg (2mmol) of compound 1 (3-bromoaniline) in 10mL of 1,2-dichloroethane, add 0.897g (4.5mmol) of benzaldehyde, 0.25mL (4.5mmol) of ice Acetic acid and 1.48g (7.0mmol) sodium triacetate borohydride, the mixed solution was stirred overnight at room temperature under nitrogen protection. After TLC detected that the reaction was complete, the reaction solution was diluted with 30 mL of 1,2-dichloroethane, and then washed with water (20 mL), saturated sodium bicarbonate (15 mL) and saturated brine (15 mL) successively, and the organic phase was washed with anhydrous sodium sulfate After drying, the organic solvent was distilled off under reduced pressure. The obtained white solid was 303 mg, yield: 75.4%. It was directly used in the next reaction...
Embodiment 2
[0077] Example 2: 5-[3-(4-benzylaminophenyl)benzylidene]-2-thio-4-thiazolone (compound 3-a)
[0078] The operation was the same as in Example 1, except that 4-bromoaniline was used instead of 3-bromoaniline to obtain 34.6 mg of a yellow solid, namely compound 3-a, with a yield of 85.9%.
[0079] 1 H NMR (300MHz, DMSO-d 6 )δ7.76-7.59(m, 5H), 7.46(d, J=9.2Hz, 2H), 7.38-7.21(m.5H), 7.05(d, J=10.4Hz, 1H), 4.51(s, 2H )
[0080] ESI-MS: m / z 403.0 (M+1, 100%)
Embodiment 3
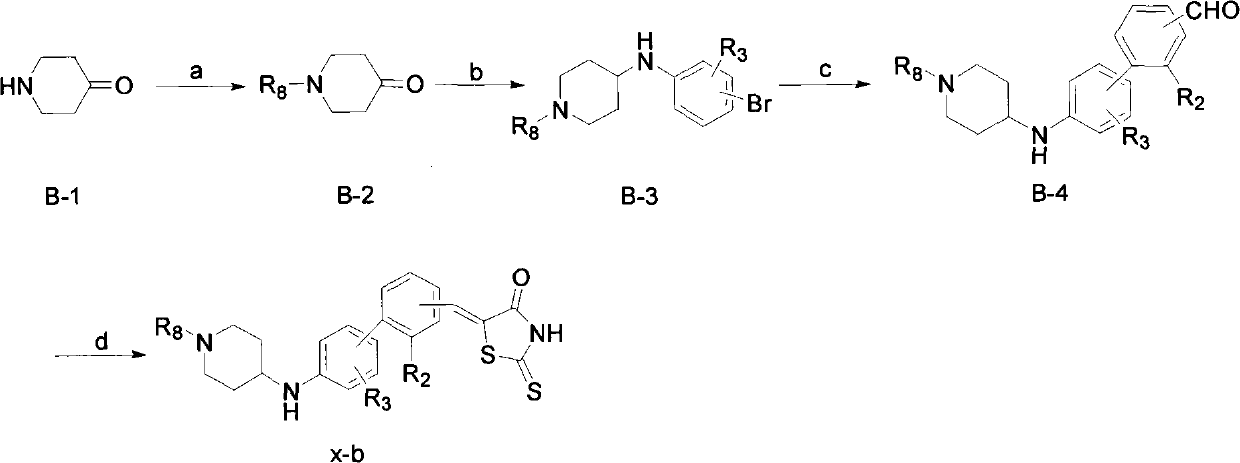
[0081] Example 3: 5-[3'-[3-(1-benzylpiperidine-4-amino)phenyl]benzylidene]-2-thio-4-thiazolone (compound 1-b)
[0082]
[0083] Referring to the preparation process of the aforementioned compound x-b, compound 1-b was prepared:
[0084] a) In a 25mL round bottom flask, under argon protection, 136mg (1.0mmoL) of compound B-1, 207mg (1.5mmoL) of potassium carbonate, 202mg (1.2mmoL) of benzyl bromide were dissolved in 15mL of acetone, Then continue to add catalytic amount of potassium iodide, and the whole reaction system was refluxed for 6 hours. After the completion of the reaction as detected by TLC, the reaction system was cooled to room temperature, potassium carbonate was removed by filtration, and the organic solvent was evaporated under reduced pressure. 163 mg of a yellow oil was obtained, that is, compound B-2, yield: 86.2%. It was directly used in the next reaction without purification.
[0085] b) 171mg (1mmol) of 3-bromoaniline was dissolved in 10mL of 1,2-dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com