Medicinal composition of radix salviae miltiorrhizae extract and application thereof
A composition and extract technology, applied in the field of pharmaceutical compositions, can solve the problems of inability to exert the overall therapeutic effect of Salvia miltiorrhiza medicinal materials, inability to reflect the compatibility and synergy of multi-component and multi-target points, limitations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
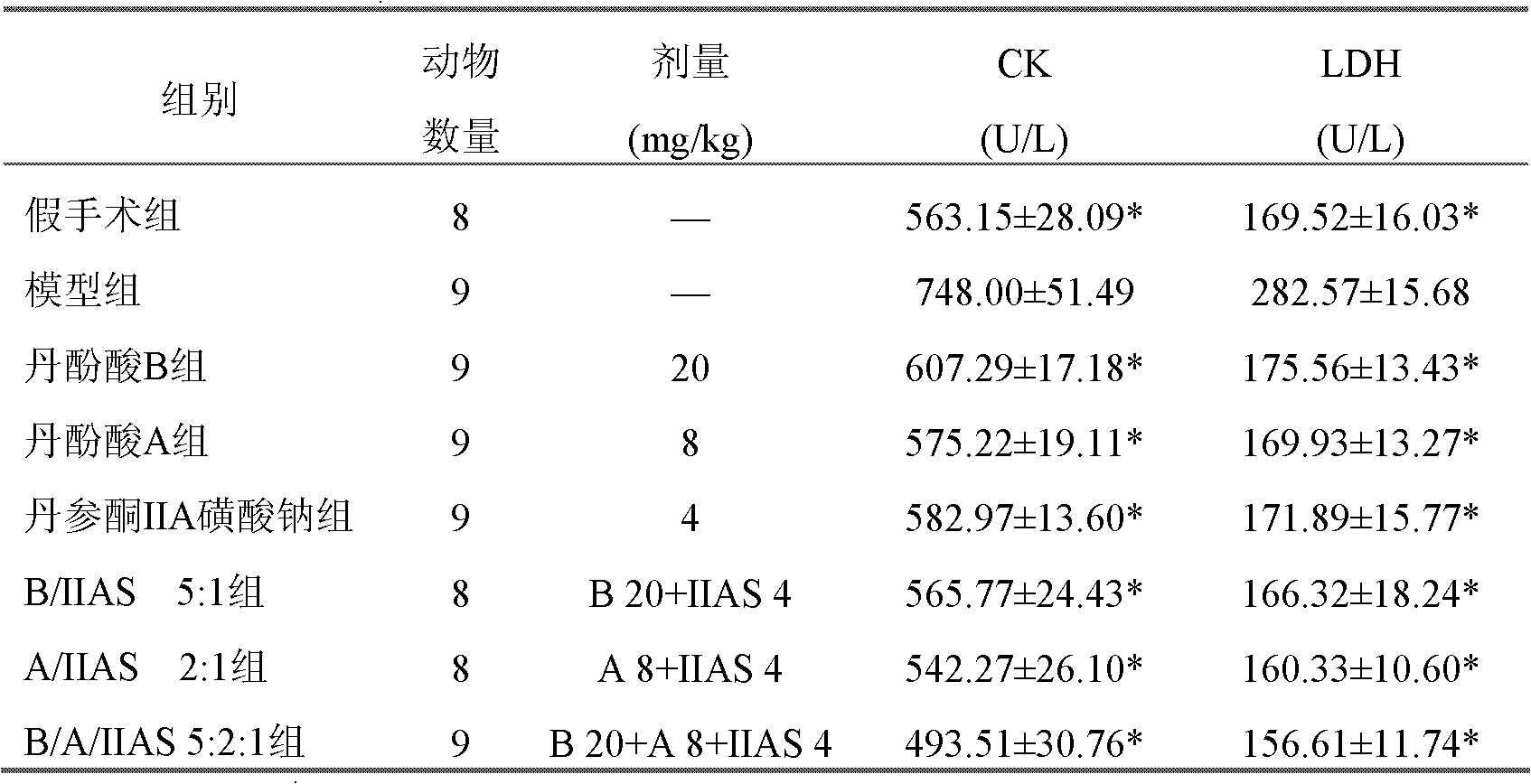
[0109] Embodiment 1: Salvianolic acid B, salvianolic acid A and tanshinone IIA sodium sulfonate (5: 2: 1) lyophilized preparation
[0110] Get 20g of salvianolic acid B, 8g of salvianolic acid A and 4g of sodium tanshinone IIA sulfonate in Example 1, add 32g sodium ascorbate, 12.5g mannitol, dissolve in 350ml distilled water, settle to 400ml, filter and sterilize, without Under the condition of bacterium, it was divided into 100 vials, freeze-dried, and sealed under nitrogen.
Embodiment 2
[0111] Embodiment 2: Salvianolic acid B, salvianolic acid A and sodium tanshinone IIA sulfonate (3:1:1) lyophilized preparation
[0112] Get 21g of salvianolic acid B, 7g of salvianolic acid A and 7g of sodium tanshinone IIA sulfonate in Example 1, add 7.4g magnesium hydrogen phosphate, 35g sodium ascorbate, 12.5g mannitol, dissolve in 250ml distilled water, stir for 20 minutes, Dilute to 300ml, filter out insoluble matter, filter and sterilize, subpackage in 100 vials under aseptic conditions, freeze-dry, and seal under nitrogen to obtain the product.
Embodiment 3
[0113] Embodiment 3: Salvianolic acid B, salvianolic acid A and sodium tanshinone IIA sulfonate (9: 3: 2) freeze-dried powder injection
[0114] Take 18g of salvianolic acid B, 6g of salvianolic acid A and 4g of sodium tanshinone IIA sulfonate, add 6g of magnesium hydrogen phosphate, 27g of sodium ascorbate, and 12.5g of mannitol, dissolve in 250ml of distilled water, stir for 20 minutes, and filter out the insoluble matter. Sterilize by filtration, freeze-dry under aseptic conditions, divide the newly prepared powdery pharmaceutical composition into 100 vials, and seal it under nitrogen to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com