Sheet-form preparation and method for producing the same
A preparation and tablet technology, applied in the field of tablet preparations, can solve problems such as difficulty in arbitrarily controlling dissolution time, anaphylactic shock pain, refrigerated storage, long time for dissolution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
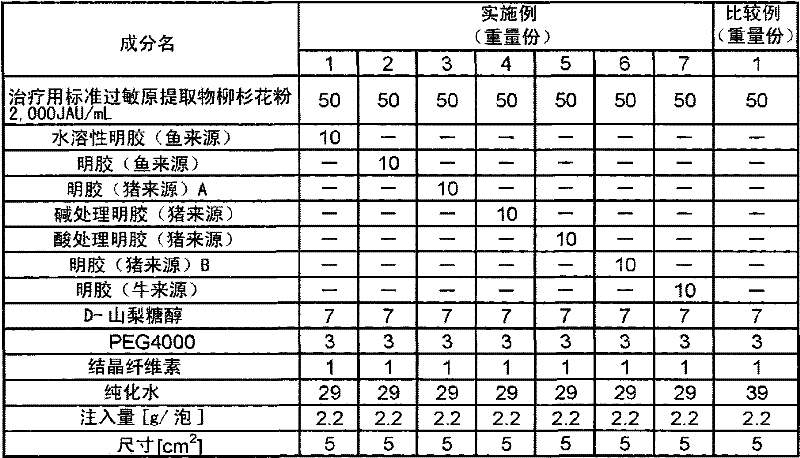
[0108] 1 part by weight of crystalline cellulose was added to 29 parts by weight of purified water, and ultrasonic dissolution and dispersion were performed. 10 parts by weight of water-soluble gelatin (average molecular weight about 100,000, hydroxyproline amount about 8.6 mol%) derived from fish (tilapia) was added thereto, and it was dissolved at a temperature of 30 to 50° C. Vibration was applied at a constant temperature of ~32°C to prepare a gelatin solution.
[0109] In addition, take 50 parts by weight of the standard allergen extract of cedar pollen 2000JAU / mL for treatment, dissolve 7 parts by weight of D-sorbitol and 3 parts by weight of PEG4000 at 2-8°C, and heat to After the temperature of 25~30℃, add all to the pre-prepared gelatin solution, mix quickly at 28~32℃, and distribute 2.2g per portion to 5cm 2 Plastic blisters (Cryomold (square) No. 3, manufactured by Sakura Finetek Japan Co., Ltd.) were cooled and solidified at 2 to 8°C for 1 day and night to obtain ...
Embodiment 2、3
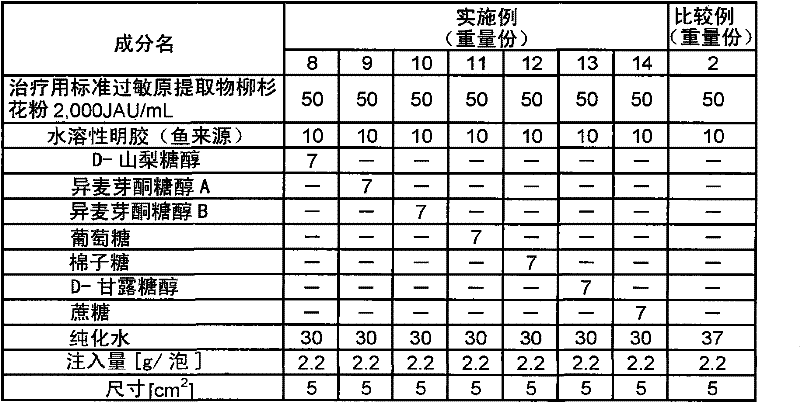
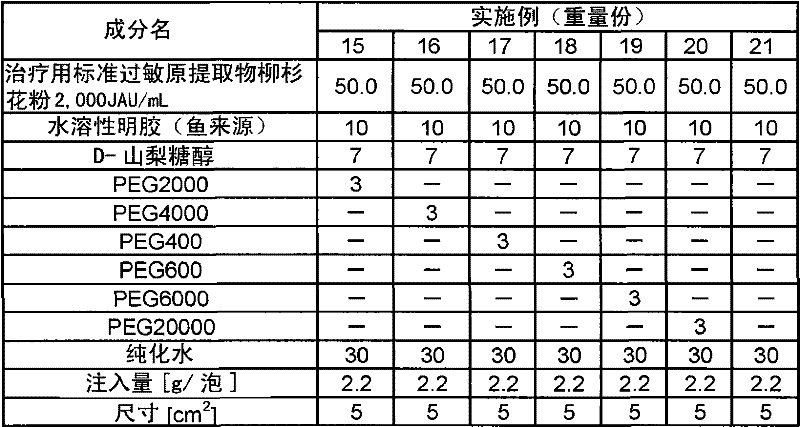
[0111] Except having adopted the composition shown in Table 1, it operated in the same procedure as Example 1, and obtained the sheet-form preparation.
[0112] Use gelatin (fish origin) (average molecular weight about 100,000, hydroxyproline amount about 8.6 mol%) in embodiment 2, use gelatin (pig origin) A (average molecular weight about 85,000, hydroxyproline amount 8.6 mol%) in embodiment 3 The amount is about 9.2 mole %).
Embodiment 4
[0117] 1 part by weight of crystalline cellulose was added to 29 parts by weight of purified water, and ultrasonic dissolution and dispersion were performed. Add 10 parts by weight of alkali-treated gelatin (porcine origin) (average molecular weight: 180,000, hydroxyproline content about 9.2 mol%), dissolve it at a temperature of 70 to 80°C, and apply it at a constant temperature of 40°C Shake to make a gelatin solution.
[0118] In addition, take 50 parts by weight of the standard allergen extract of cedar pollen 2000JAU / mL for treatment, dissolve 7 parts by weight of D-sorbitol and 3 parts by weight of PEG4000 at 2-8°C, and heat to After a temperature of 40°C, add all to the pre-prepared gelatin solution, mix quickly at 40°C, and dispense 2.2g per portion into 5cm 2 The plastic blister (Cryomold (square) No. 3, manufactured by Sakura Finetek Japan Co., Ltd.) was cooled and solidified at 2 to 8°C for 1 day and night to obtain a sheet-like preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com