Preparation technology of aztreonam for injection
A preparation process, the technology of aztreonam, is applied in the direction of making medicines into special physical or taking forms of devices, antibacterial drugs, powder delivery, etc., and can solve problems such as poor antibacterial effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The solutions of the present invention will be further described below in conjunction with specific examples. For the convenience of expression, the compounds mentioned are represented by their common names.
[0022] prescription
[0023] Aztreonam / Arginine 500 g (as aztreonam)
[0024] Packing 1000 bottles
[0025] Preparation Process
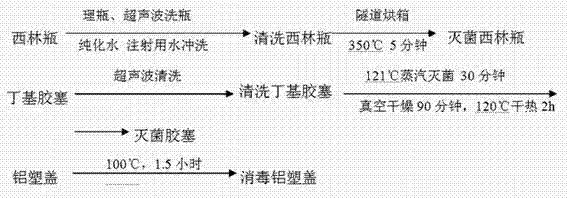
[0026] 1. Cleaning and sterilization of vials, butyl rubber stoppers and aluminum-plastic caps
[0027] Such as figure 1
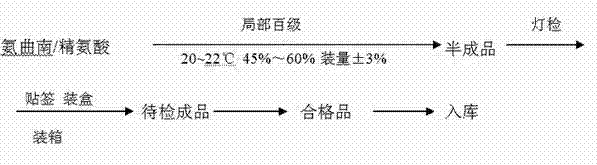
[0028] 2. Packing
[0029] Such as figure 2
[0030] It should be noted that the above descriptions are only preferred embodiments of the present invention, and are not intended to limit the scope of the present invention. Any modifications, equivalent replacements and improvements made within the spirit and principles of the present invention , should be included within the protection scope of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com