Lamivudine solid dispersion, and preparation method, pharmaceutical composition and use of the dispersion
A solid dispersion, lamivudine technology, applied in the field of medicine, can solve the problems of high production cost, many influencing factors, operator injury and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
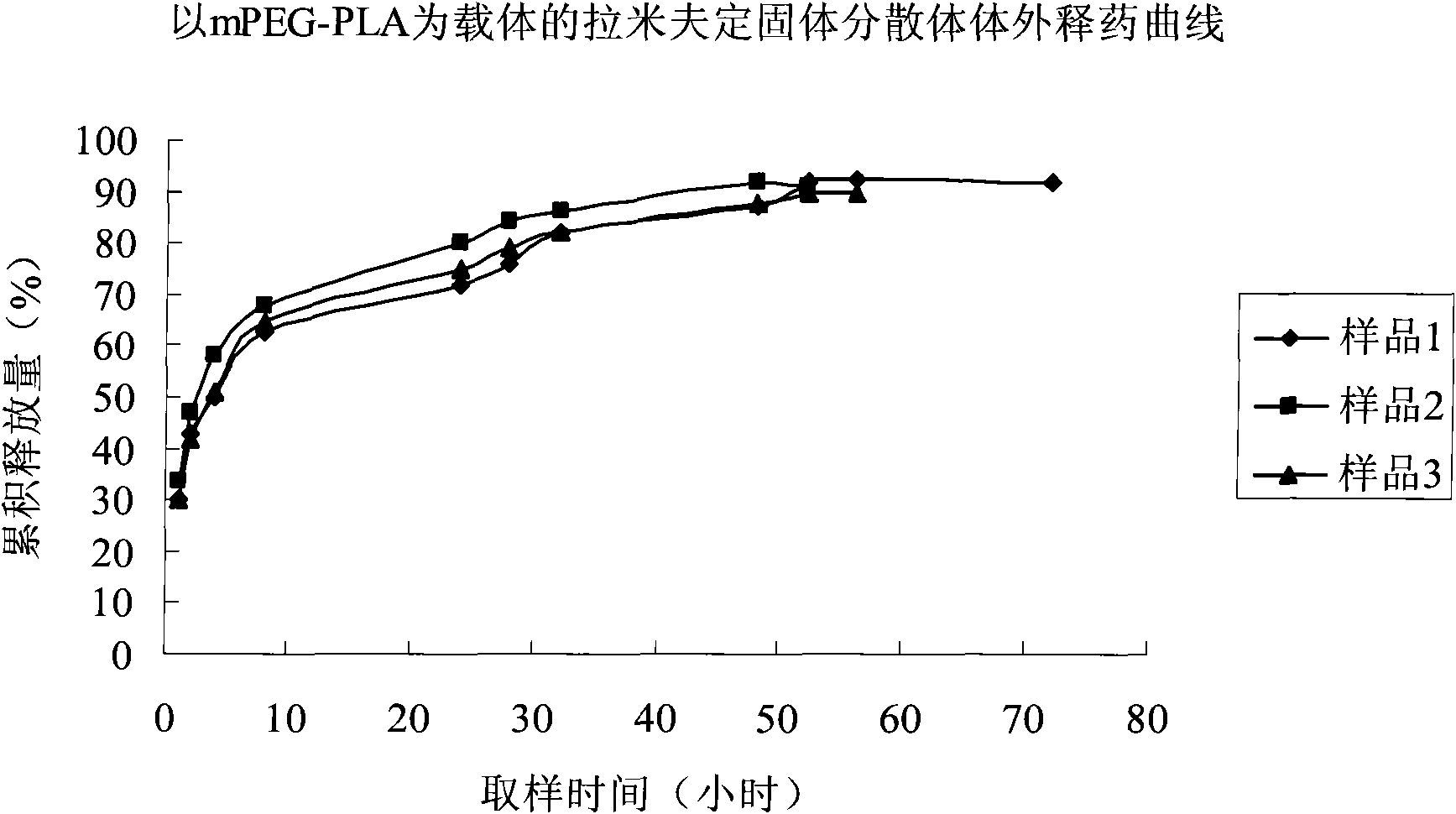
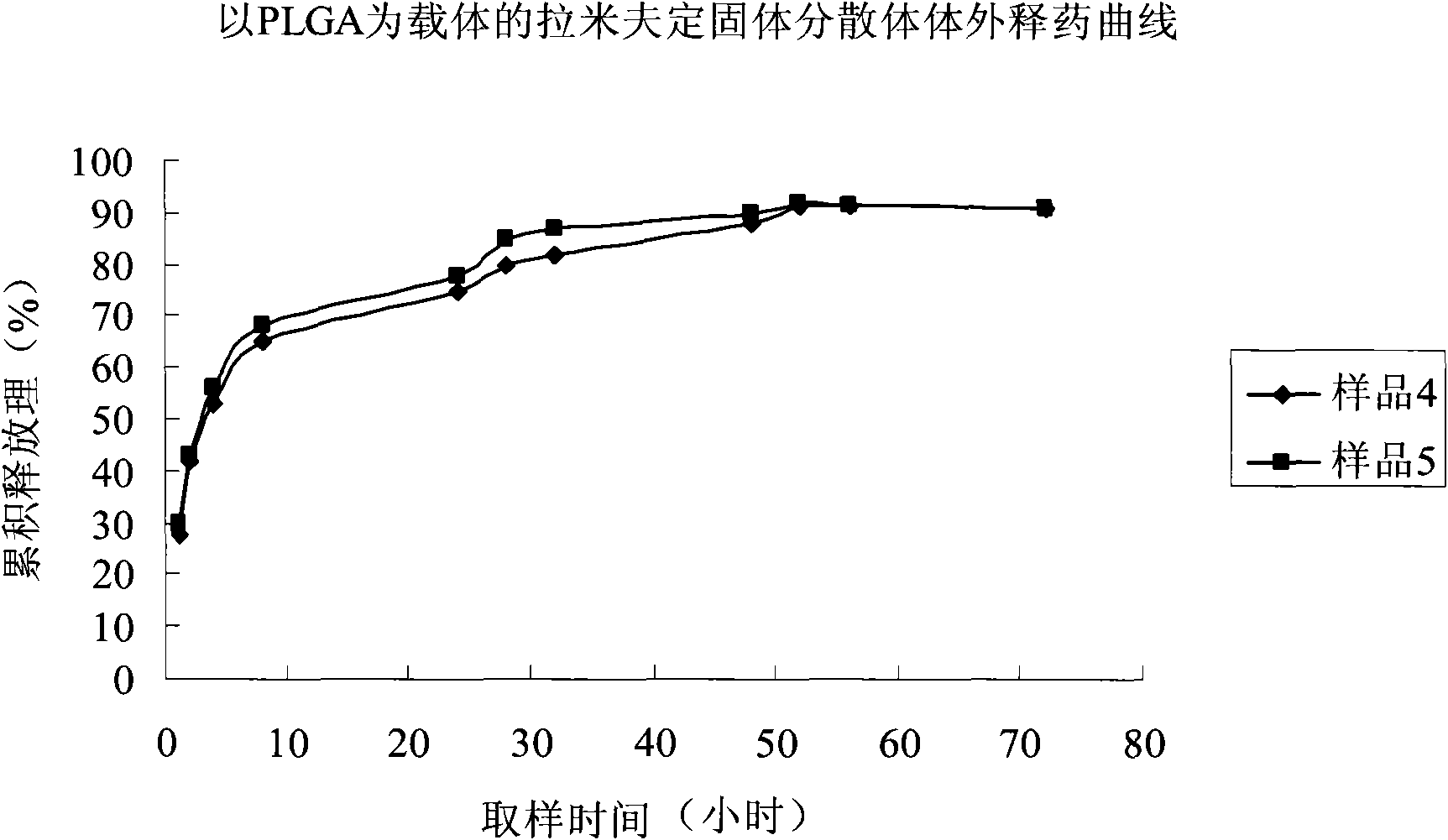
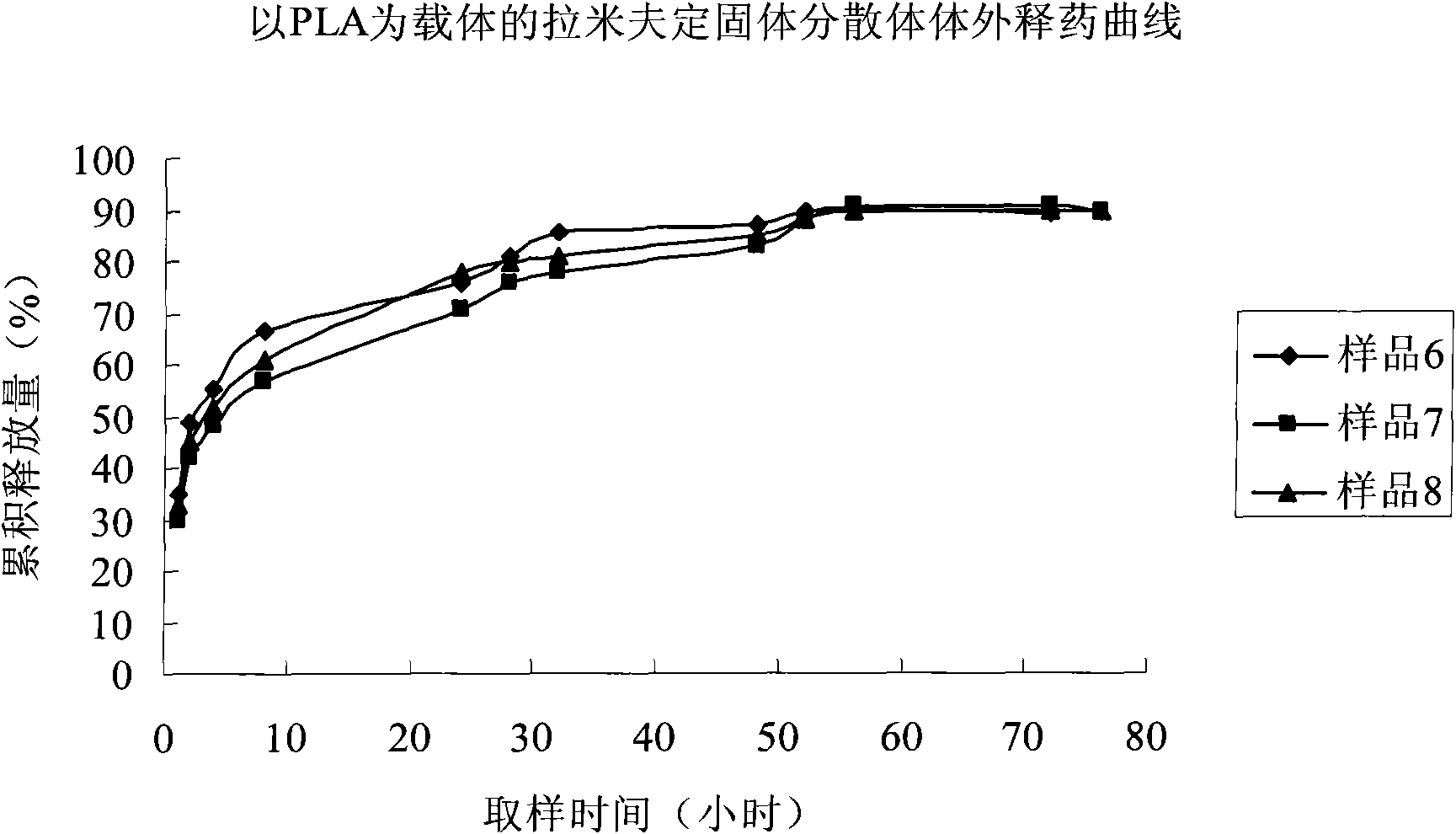
Image
Examples
Embodiment 1
[0059] Feeding amount:
[0060] Lamivudine 6.0g
[0061] mPEG-PLA (Mw=750 / 10000) 6.0g
[0062] Triethyl citrate 1.8g
[0063] Cholesterol 0.12g
[0064] Povidone K30 0.08g
[0065] Preparation method: Take mPEG-PLA, lamivudine, triethyl citrate, cholesterol, povidone K30, mix well, heat to melt, shear until evenly mixed, cool, pulverize, collect solid dispersion, namely have to.
[0066] Lamivudine content: 39.7%.
[0067] In this example, the molecular structural formula of mPEG-PLA is
[0068]
[0069] Among them: m≈17, n≈138.
Embodiment 2
[0071] Feeding amount:
[0072] Lamivudine 8.4g
[0073] mPEG-PLA (Mw=2000 / 5000) 5.6g
[0074] Preparation method: Take mPEG-PLA and lamivudine, mix them evenly, heat until melted, shear until mixed evenly, cool, pulverize, and collect the solid dispersion to obtain the product.
[0075] Lamivudine content: 60.2%.
[0076] In this example, the molecular structural formula of mPEG-PLA is
[0077]
[0078] Among them: m≈45, n≈69.
Embodiment 3
[0080] Feeding amount:
[0081] Lamivudine 0.8g
[0082] mPEG-PLA (Mw=2000 / 80000) 8.0g
[0083] Preparation method: Take mPEG-PLA and lamivudine, mix them evenly, heat until melted, shear until mixed evenly, cool, pulverize, and collect the solid dispersion to obtain the product.
[0084] Lamivudine content: 8.5%.
[0085] In this example, the molecular structural formula of mPEG-PLA is
[0086]
[0087] Among them: m≈45, n≈1111.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com