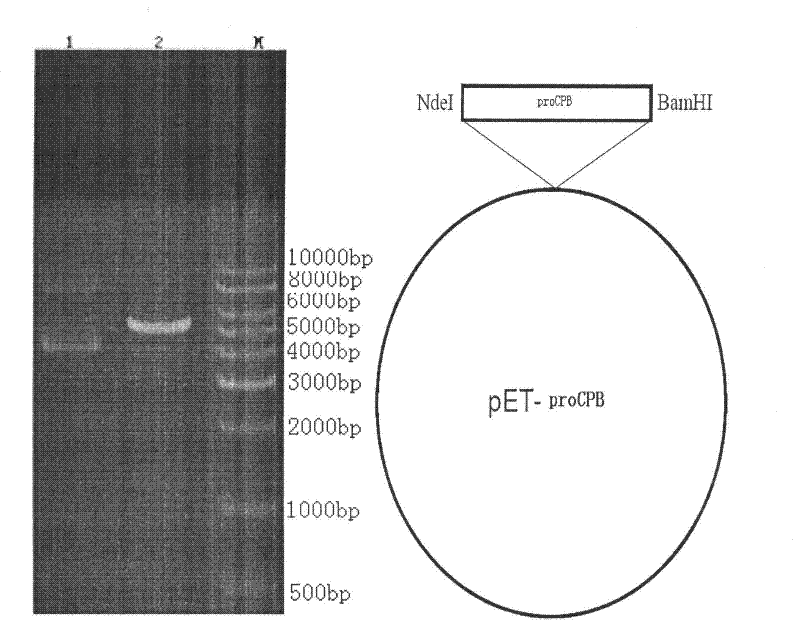
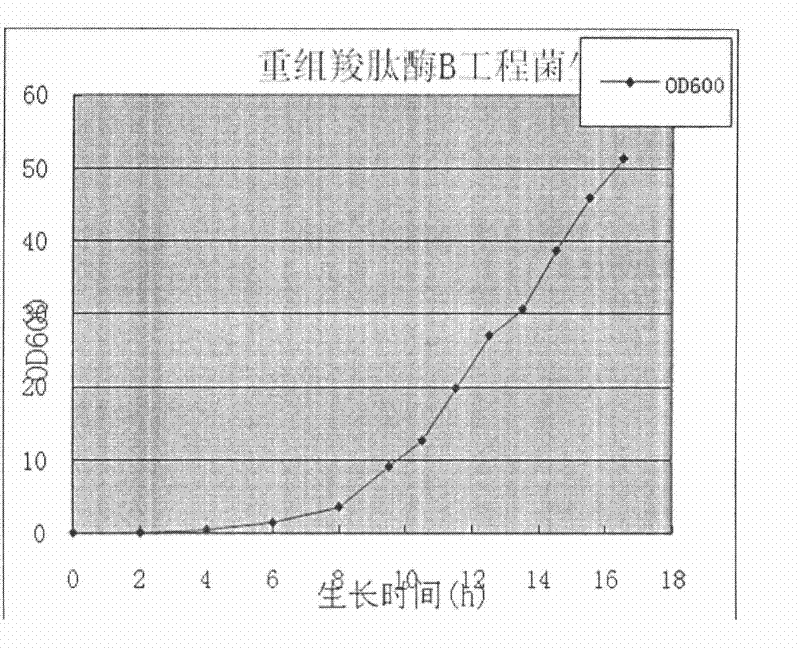
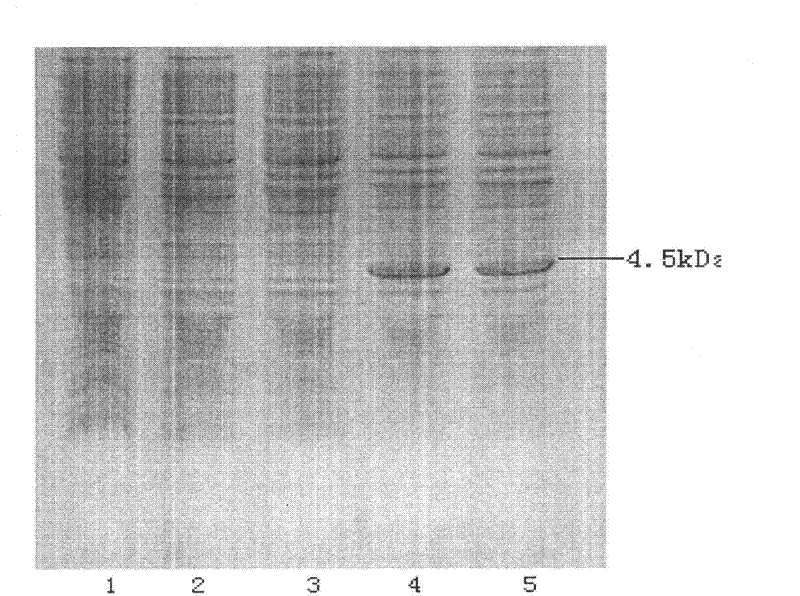
Preparation method of recombinant carboxypeptidase B
A technology of carboxypeptidase and enzyme cleavage site, which is applied in the field of preparation of recombinant carboxypeptidase B, and can solve problems such as the risk of pharmaceutical proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 3
[0053] Example 3 Carboxypeptidase B Purification
[0054] After the fermentation, the cells were collected by centrifugation, and the crushing buffer (25mmol / L Tis-HCL+5mmol / L EDTA, pH7.5) was added at a weight-to-volume ratio of 1:10. The inclusion body precipitate was collected, and the weight loss of the inclusion body was 30g / L fermentation broth. Add the precipitate to washing buffer (2 mol / L urea + 1.5% Triton) at a weight-to-volume ratio of 1:10, stir magnetically at room temperature for 1 hour, and wash the precipitate collected by centrifugation twice with washing buffer. The inclusion body was then dissolved overnight with inclusion body dissolution buffer (8mol / L urea+10mmol / LEDTA+25mmol / L Tis-HCL, pH7.5) at a weight-to-volume ratio of 1:10. The dissolved inclusion bodies were centrifuged and ultrafiltered to remove impurities (such as Figure 4 shown), diluted to a protein concentration of 0.2mg / ml, in refolding buffer (0.2mol / L urea+10mmol / LEDTA+25mmol / L Tis-HCL...
example 4
[0055] The activity measurement of example 4 carboxypeptidase
[0056] The activity of carboxypeptidase B was determined by hydrolyzing hippuronyl-L-arginine at 25°C and pH 7.65,
[0057] Buffer: 25 mM Tris HCl buffer containing 100 mM NaCl at 25°C, pH 7.65.
[0058] Substrate solution: 1.0 mM hippurinyl-L-arginine solution.
[0059] Carboxypeptidase B enzyme solution: (prepared when used, containing 4-8 enzyme activity units / ml water)
[0060] Record the increase in absorption value within 5 minutes ( Figure 6 ), the maximum absorbance / time is obtained using the maximum linear velocity of the assay and blank.
[0061]
[0062] df: dilution factor
[0063] It was determined that the purified recombinant carboxypeptidase B had an activity of 98 units per milligram.
[0064]
[0065]
[0066]
[0067]
[0068]
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com