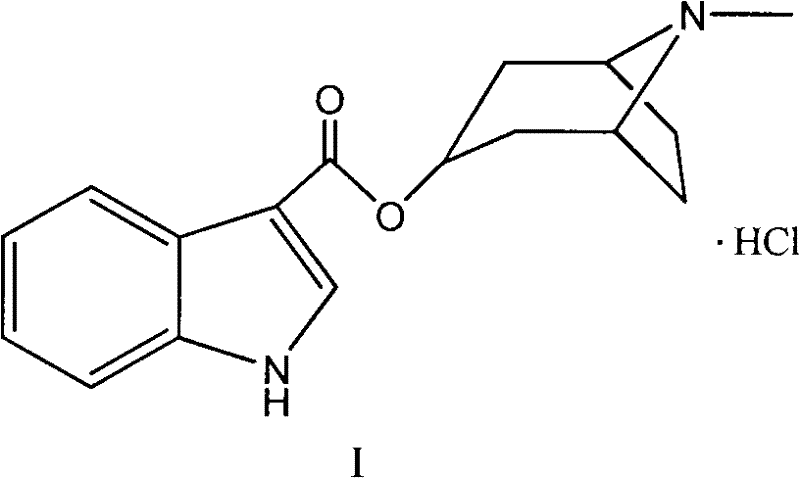
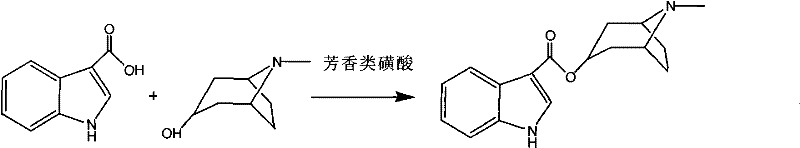
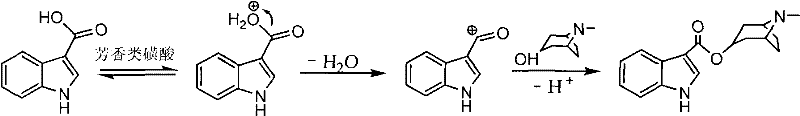
Synthetic method of tropisetron and prepare method of hydrochloric acid tropisetron
A synthetic method, the technology of tropisetron, applied in the field of chemical pharmaceuticals, to achieve the effects of simplified synthesis steps, less pollution, and easy preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a simple method for preparing high-purity tropisetron hydrochloride. The method first realizes direct esterification synthesis of tropisetron through suitable catalyst and reaction conditions (specific conditions as described above), and then through acidification Step (including extracting the tropisetron obtained by esterification into an aqueous mineral acid solution), alkali adjustment step (including adjusting the pH value to 9-10, so that the crude product of tropisetron is precipitated from water), refining step (including using hydrochloric acid ethanol solution for salt formation and crystallization) to obtain Tropisetron Hydrochloride.
[0050] After the reaction for synthesizing tropisetron is completed, tropisetron is placed in an organic solvent, and the tropisetron is extracted into an aqueous solvent through an acidification step, preferably using an aqueous mineral acid solution to extract tropisetron. The aqueous inor...
Embodiment 1
[0068] Add indole-3-carboxylic acid (20 g, 0.124 mol), p-toluenesulfonic acid (0.75 g, 0.00434 mol), ethyl acetate (230 ml), freshly activated 4A molecular sieves (0.5-1.0 mm) (3 g) In a 500ml three-necked reaction flask equipped with a thermometer and a reflux condenser, start to heat up after stirring evenly, and the temperature is controlled at 75-77°C, then slowly add tropinol (19.3 grams, 0.137mol) dropwise, and reflux after the dropwise addition React for 10 hours.
[0069] Stop the reaction, extract the product three times with 100ml of 1mol / L hydrochloric acid in the organic layer, combine the aqueous phases, and wash once with 50ml of ethyl acetate. The aqueous phase was adjusted to pH 9-10 with 4 mol / L sodium hydroxide aqueous solution, and a light yellow solid was precipitated. The filter cake was suction filtered and washed with distilled water until neutral, and dried under reduced pressure to obtain 32.7 g of crude tropisetron. Dissolve the above crude product i...
Embodiment 2
[0071] Indole-3-carboxylic acid (20 g, 0.124 mol), benzenesulfonic acid (0.791 g, 0.005 mol), ethyl acetate (230 ml), freshly activated 4A molecular sieves (0.5-1.0 mm) (3 g) were added to In the 500ml three-necked reaction flask equipped with a thermometer and a reflux condenser, start to heat up after stirring evenly, and the temperature is controlled at 75°C-77°C, then slowly add tropinol (19.3 grams, 0.137mol) dropwise, and reflux after the dropwise addition React for 11 hours.
[0072] Stop the reaction, extract the product three times with 100ml of 1mol / L hydrochloric acid in the organic layer, combine the aqueous phases, and wash once with 50ml of ethyl acetate. The aqueous phase was adjusted to a pH of 9-10 with 4 mol / L sodium hydroxide aqueous solution, and a yellow solid was precipitated. The filter cake was suction filtered and washed with distilled water until neutral, and dried under reduced pressure to obtain 33.1 g of crude tropisetron. Dissolve the above crude...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com