Terpyridyl derivative with electroluminescent and electrochromic characteristics and complex thereof
An electrochromic and terpyridine technology, applied in the field of organic electronics, can solve the problems of low device service life, limited practical application, poor thermal stability, etc., and achieve the effect of sensitive electrochromic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
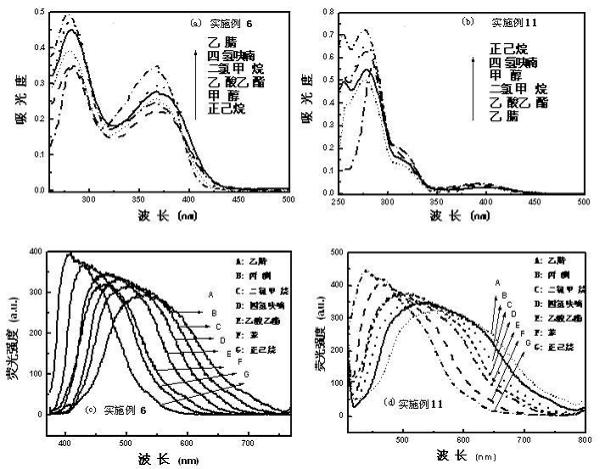
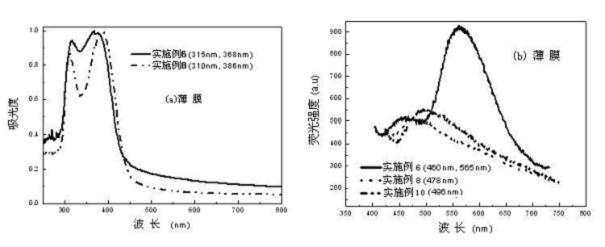
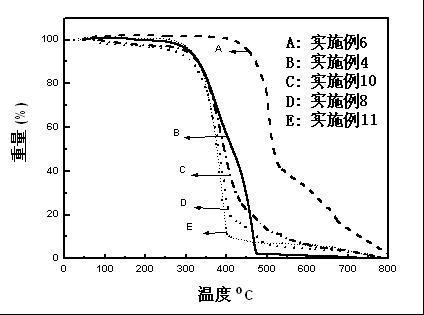
Image
Examples
Embodiment 1
[0071] Embodiment 1: the synthesis of 4-(4'-nitro)-phenyl terpyridine
[0072]
[0073] [1] Synthesis of Intermediate 1: Add 10.8 mmol p-nitrobenzaldehyde to 100 mL MeOH, then add equimolar 2-acetylpyridine (10.8 mmol) and NaOH (2%, 0.44 g, 22 mL) and stir for 2 hours, A solid powder precipitated out. Rinse with water and methanol, filter and dry to obtain solid powder with a yield of 89.3%.
[0074] [2] Synthesis of Intermediate 2: Dissolve elemental iodine 5.06g (20mmol) in hot pyridine (30mL), add 2-acylpyridine (2.42g, 20.0mmol) under argon protection, and stir at 80°C 4h. After cooling, the solid product was filtered and rinsed 3 times with pyridine. The solid product was then decolorized by boiling 2 L of ethanol containing activated carbon. Filtrate while hot to obtain a yellow-green solid, and dry to obtain 4.38 g of a yellow-green solid with metallic luster, with a yield of 67.2%. Melting point: 227-228°C.
[0075] [3] Synthesis of 4-(4'-nitro)-phenyl terpyri...
Embodiment 2
[0076] Embodiment 2: the synthesis of 4-(4'-formyl)-phenyl terpyridine
[0077] Using a method similar to that in Example 1, only 4-nitrobenzaldehyde in step 1 was changed to 1,4-terephthalaldehyde according to the obtained compound, and the yield was 42.3%. Mass spectrum: m / z (EI) 337.12 (M + , 100%). 1 H NMR (CDCl 3 ; 400MHz; TMS): δ, ppm 7.12 (m, 2H, Ar-H), 7.66 (m, 4H, Ar-H), 7.87 (d, 2H, Ar-H), 8.46 (m, 4H, pyr- H), 8.59 (d, 2H, o-pyr-H), 9.87 (s, 1H, CHO).
[0078] The molecular formula of the compound obtained in this embodiment is,
[0079]
Embodiment 3
[0080] Embodiment 3: the synthesis of 4-(4'-carboxy)-phenyl terpyridine
[0081] Put 1g of the compound obtained in Example 2 into a three-necked flask, add 20mL of methanol and a small amount of tetrabutylammonium bromide, stir, and then dropwise add KMnO 4 Aqueous solution (2g KMnO 4 ), the dropwise addition was completed and the reaction was continued for 24 hours, then the reaction solution was suction filtered until clarified, and the pH value of the filtrate was adjusted until solids were precipitated, then filtered and dried. 0.76 g of product was obtained, a 72% yield. Mass spectrum: m / z (EI) 353.14 (100%, M + ). 1 H NMR (CDCl 3 ; 400MHz; TMS): δ, ppm 7.12 (m, 2H, o-pyr-H), 7.69-7.66 (m, 4H, Ar-H), 8.19 (d, 2H, o-benzoic acid), 8.46 (m , 4H, pyr-H), 8.59 (d, 2H, o-pyr-H). The molecular formula of the compound obtained in this embodiment is,
[0082]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com