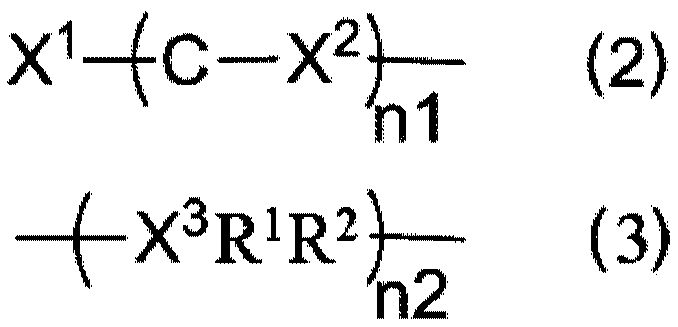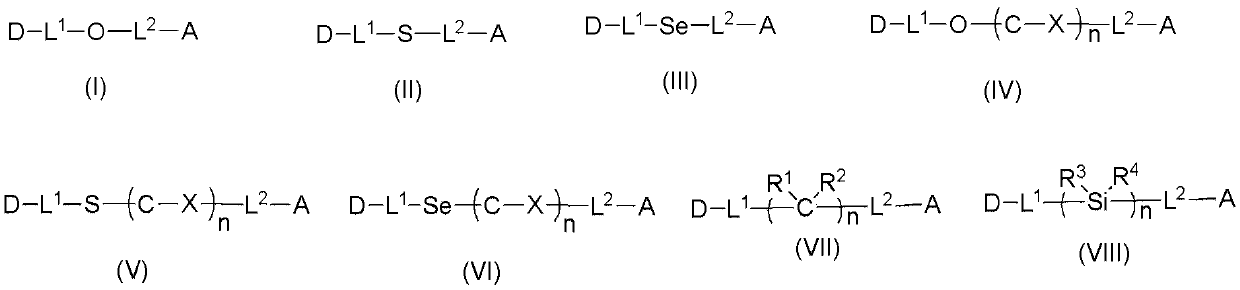Organic compound and organic electroluminescent device containing same
A compound and unsubstituted technology, applied in the field of organic compounds, can solve problems such as efficiency roll-off, high device turn-on voltage, and restrictive development, and achieve the effects of reducing efficiency roll-off, reducing driving voltage, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0109] Synthesis Example 1: Synthesis of M1-1
[0110]
[0111] Synthesis of M1-1:
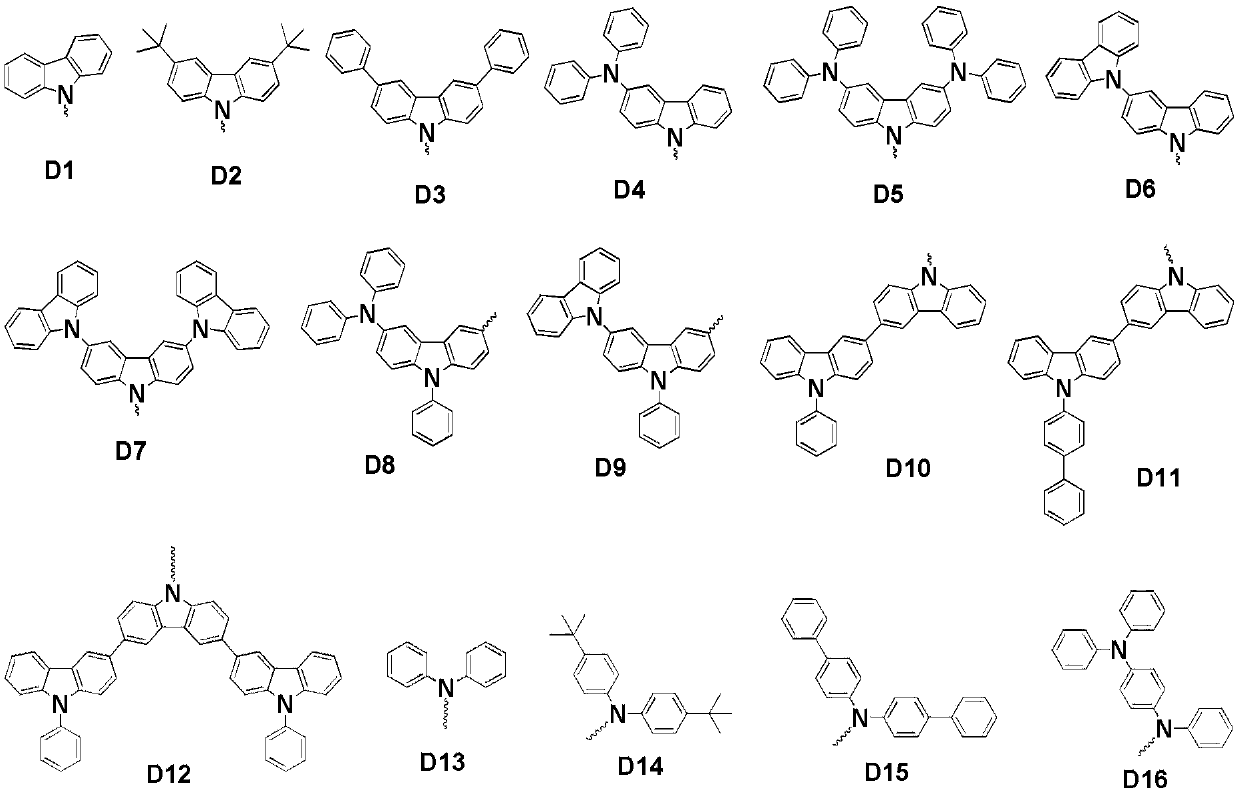
[0112] Add 600mg of sodium hydride (60%, 15mmol) into a 250mL dry two-necked flask, then add 20mL of dry N,N-dimethylformamide (DMF), and stir for 5min. After that, 20 mL of DMF solution in which 4.11 g of 3-(3,6-diphenyl-9H-carbazole)phenol (10 mmol) was dissolved was added dropwise, and stirring was continued for 1 h after the addition was completed. 2.67 g of 2-chloro-4,6-diphenyl-1,3,5-triazine (10 mmol) was dissolved in 20 mL of dry DMF, and then added dropwise to the reaction system. After the dropwise addition was completed, the temperature was raised to 100° C. and stirring was continued for 12 h. After the reaction system dropped to room temperature, it was poured into 200 mL of ice water, and the precipitate was collected by filtration and dried in vacuum. The crude product was separated by column chromatography to obtain 5.9 g with a yield of 92%. The molecular ion mass deter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com