Strontium ranelate orally disintegrating tablets and preparation method thereof
A technology of orally disintegrating tablets and strontium ranelate, applied in the field of orally disintegrating strontium ranelate tablets and its preparation, can solve problems such as not being suitable for taking, and achieve extensive social and economic benefits, fast absorption, and bioavailability high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Prescription: (mass ratio)
[0025]
[0026] Preparation:
[0027] According to the ratio shown in the prescription, pass microcrystalline cellulose, mannitol and crospovidone through a 40-mesh sieve, pass strontium ranelate and magnesium stearate through a 60-mesh sieve, and pass citric acid and aspartame through a 100-mesh sieve. Mesh sieve. Then add them all into the mixing granulator and mix for 15 minutes until they are evenly mixed. Determination of intermediate content. According to the content, each tablet contains 2 g of strontium ranelate and is pressed into tablets to obtain strontium ranelate orally disintegrating tablets.
[0028] Tests and results:
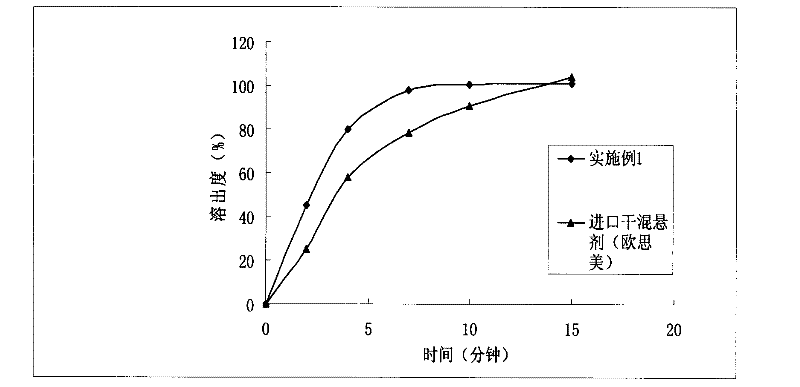
[0029] The orally disintegrating tablet prepared in Example 1, its in vitro release test method is as follows: select the II method of the Chinese Pharmacopoeia 2010 edition appendix XC dissolution test method, take 1000ml of hydrochloric acid solution (9→1000) as the dissolution medium, and the rotatin...
Embodiment 2
[0032] Prescription: (mass ratio)
[0033]
[0034] Preparation:
[0035] The ratio shown in the prescription, pass microcrystalline cellulose, lactose and croscarmellose sodium through a 40-mesh sieve, pass strontium ranelate and magnesium stearate through a 60-mesh sieve, pass citric acid and aspartame Pass through a 100 mesh sieve. Then add them all into the mixing granulator and mix for 15 minutes until they are evenly mixed. Determination of intermediate content. According to the content, each tablet contains 2 g of strontium ranelate and is pressed into tablets to obtain strontium ranelate orally disintegrating tablets.
[0036] Tests and results:
[0037] The orally disintegrating tablet prepared in Example 2, its in vitro release test method is as follows: select the II method of the Chinese Pharmacopoeia 2010 edition appendix XC dissolution test method, take 1000ml of hydrochloric acid solution (9 → 1000) as the dissolution medium, and the rotating speed is 50 ...
Embodiment 3
[0039] Prescription: (mass ratio)
[0040]
[0041] Preparation:
[0042] The ratio shown in the prescription, pass microcrystalline cellulose, mannitol and crospovidone through a 40-mesh sieve, pass strontium ranelate, magnesium stearate and microsilica gel through a 60-mesh sieve, pass citric acid and stevioside through a 100-mesh sieve Mesh sieve. Then add them all into the mixing granulator and mix for 15 minutes until they are evenly mixed. Determination of intermediate content. According to the content, each tablet contains 2 g of strontium ranelate and is pressed into tablets to obtain strontium ranelate orally disintegrating tablets.
[0043] Tests and results:
[0044] The orally disintegrating tablet prepared in Example 3, its in vitro release test method is as follows: select the II method of the Chinese Pharmacopoeia 2010 edition appendix XC dissolution test method, take 1000ml of hydrochloric acid solution (9→1000) as the dissolution medium, and the rotatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com