Method for predicating vapor pressure of liquid phase of organic substance by quantitative structure-activity relationship model
A quantitative structure-activity relationship and model prediction technology, applied in special data processing applications, instruments, electrical digital data processing, etc., to achieve good fitting ability, high reliability, and easy mechanism interpretation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
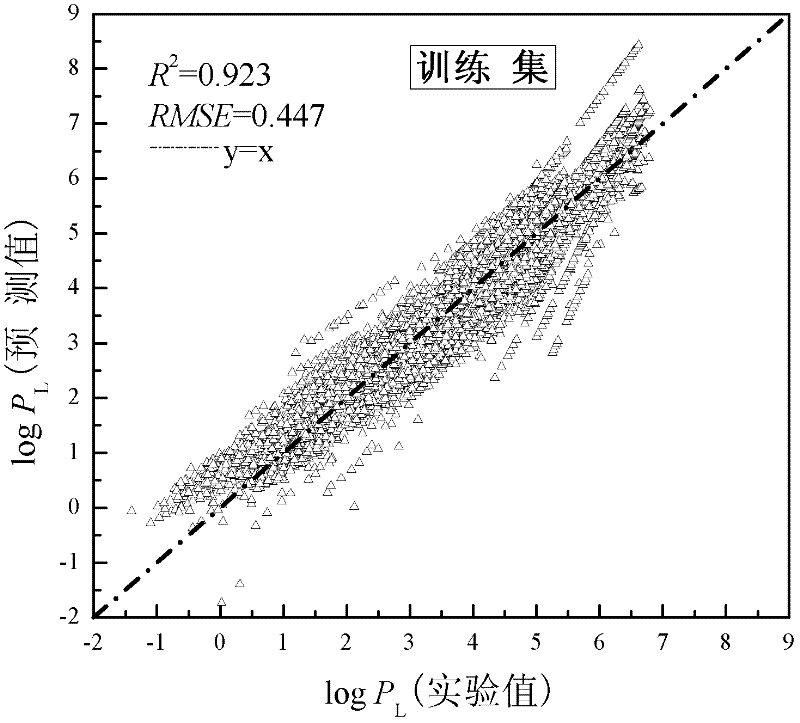
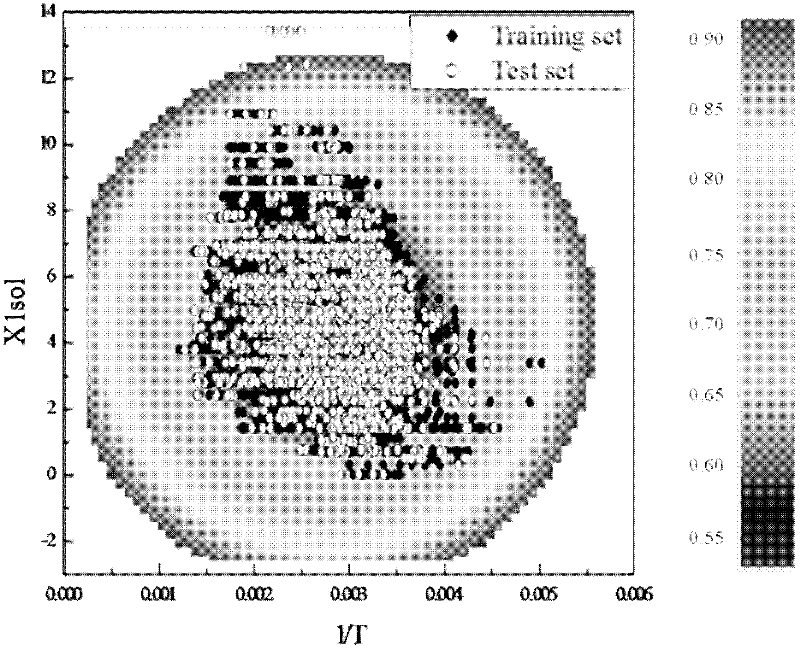
[0023]Given a compound, methyl cinnamate, which contains a benzene ring structure and an oxygen atom. To predict its vapor pressure at 270K, 285K, 298K, 310K, 330K temperature. First of all, according to the structural information of methyl cinnamate, use the MOPAC2009 software to optimize its structure, and the value of μ can be calculated to be 5.574; the values of XOA, X1sol and GATS1v are calculated by Draogon software, and their values are 0.734, respectively. 5.826 and 2.156; NHD, MFP and JRNCG were calculated by Discovery Studio software, and their values were 0, 0.155 and 0.334, respectively. Then, through the obtained application domain characterization diagram, it can be concluded that the compound falls within the scope of the application domain, so this model can be used for prediction. Substitute T=270K, 285K, 298K, 310K, 330K and μ=5.574, XOA=0.734, X1sol=5.826, GATS1v=2.156, NHD=0, MFP=0.155, JRNCG=0.334 respectively into the obtained linear relational fo...
Embodiment 2
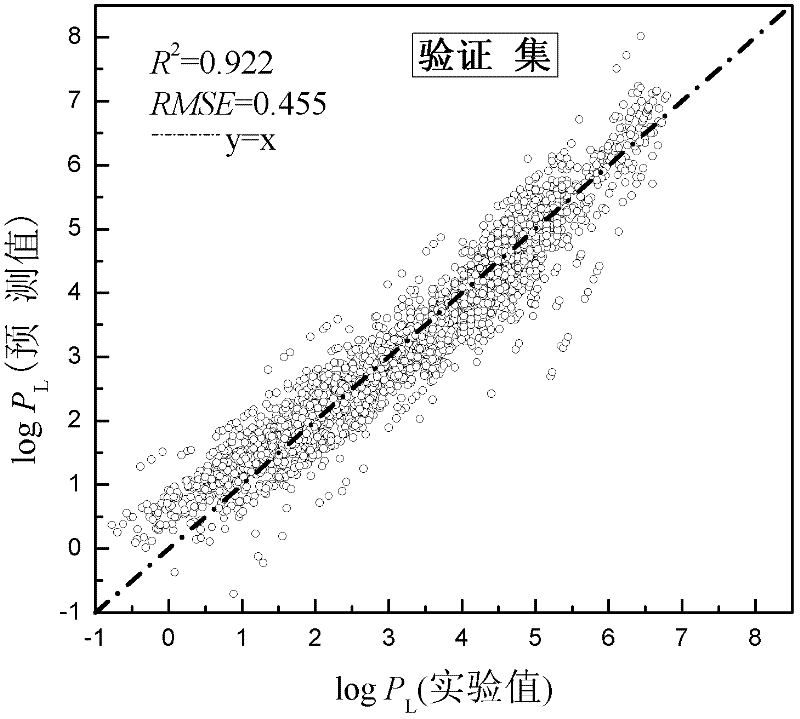
[0028] Given a compound propanol with more experimental data. Predict its vapor pressure at 298K, 303K, 308K, 313K, 318K, 323K and compare with the experimental value. According to the structural information of propanol, the values of μ, NHD, MFP, JRNCG, XOA, X1sol and GATS1v were calculated as 2.177, 1, 0.229, 0.805, 0.854, 1.914 and 1.333, respectively, using MOPAC2009, Dragon and Discovery Studio software. Through the application domain characterization diagram, it can be concluded that propanol falls within the scope of the application domain, and this model can be used for prediction. Substituting T=298K, 303K, 308K, 313K, 318K, 323K and the above-mentioned descriptor data into the linear relational expression obtained by modeling, the logP can be obtained L At T=298K, 303K, 308K, 313K, 318K, and 323K, they are 3.307, 3.453, 3.593, 3.730, 3.861, and 3.989, respectively. and its logP at the corresponding temperature L Compared with the experimental data values 3.459...
Embodiment 3
[0030] Given the compound n-hexacane. Predict its liquid phase vapor pressure at higher temperatures 402K, 423K, 432K, 452K, 462K. According to the structural information of n-hexacane, the values of μ, NHD, MFP, JRNCG, XOA, X1sol and GATS1v were calculated as 0.063, 0, 0, 0.045, 0.729, respectively, using MOPAC2009, Dragon and Discovery Studio software. 13.414 and 3.074. Substituting T=402K, 423K, 432K, 452K, 462K and the calculated descriptor values into the linear relational formula obtained by modeling, it can be obtained that n-hexacane is at T=402K, 423K, 432K, 452K, and 462K. logP L The values are 0.125, 0.449, 0.579, 0.847 and 0.973, respectively. But with the logP at the corresponding temperature L Compared with the experimental values -0.228, 0.430, 0.745, 1.330, and 1.603, the difference is relatively large. However, through the characterized application domain, it can be found that the value of the compound X1sol (13.414) exceeds the range of the chara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com