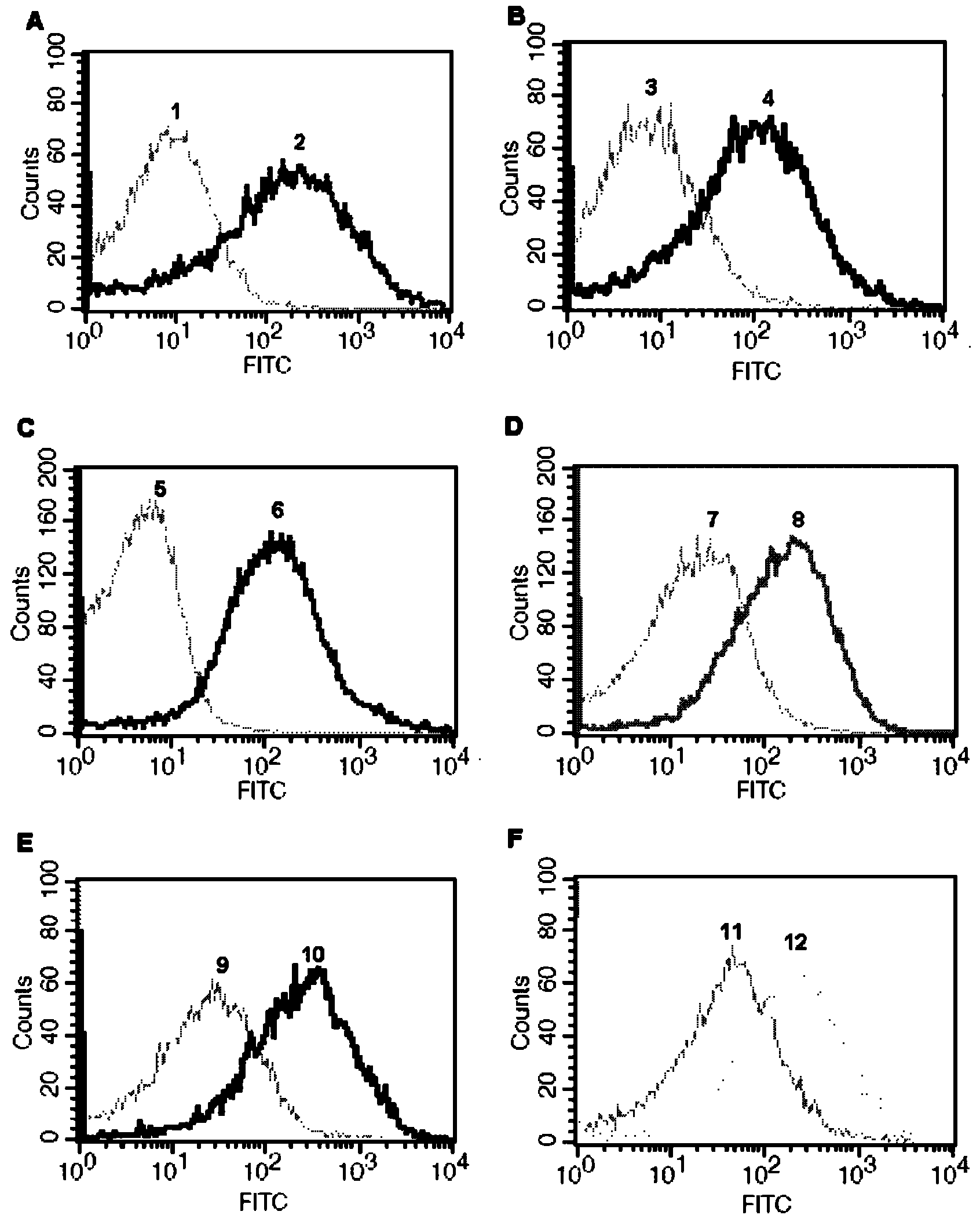
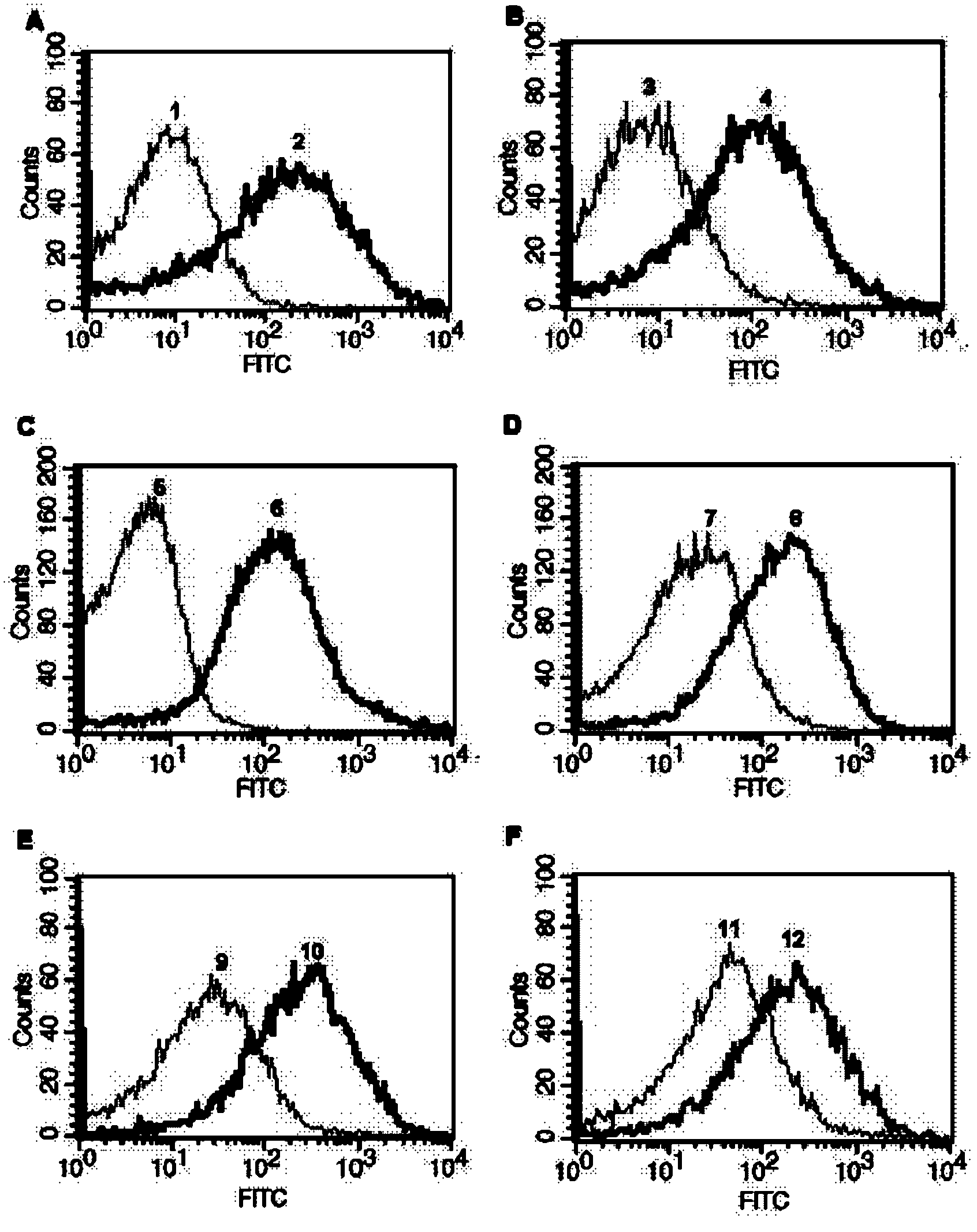
Intracellular protein detection method by flow cytometry
A flow cytometry, intracellular protein technology, applied in biological testing, material testing, etc., to achieve the effect of strong surface adsorption, simple preparation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Take the expression of actin (β-actin) in the human liver cancer HepG2 cell line as an example to illustrate.
[0041] 1. Preparation of Silica Nanoparticles
[0042] (1) Preparation of surface aminated silica nanoparticles:
[0043] adopt first Law( W, Fink A, Bohn E.J Colloid Interface Sci, 1968, 26~62.) prepare 100~120nm silica nanoparticles; then add about 1g of silica nanoparticles to 30ml of anhydrous toluene, stir at room temperature, and then Slowly add 2.5ml of γ-aminopropyltriethoxysilane (ATES) dropwise, continue to stir for 1h, then raise the temperature to 95°C, reflux for 10h, wash with isopropanol ultrasonically for 3 times, and vacuum dry at 60°C for 10h to obtain about 1g surface aminated silica nanoparticles;
[0044] (2) Preparation of surface carboxylated silica nanoparticles:
[0045] adopt first Law( W, Fink A, Bohn E.J Colloid Interface Sci, 1968, 26~62.) Prepare 100~120nm silica nanoparticles; then uniformly disperse 0.01M s...
Embodiment 2
[0056] Example 2: Take the expression of protein kinase B (Akt2) in human breast cancer MCF-7 cell line as an example to illustrate.
[0057] 1. the preparation of silicon dioxide nanoparticle (same as embodiment 1)
[0058] 2. Preparation of nanoparticles with specificity for binding intracellular proteins
[0059] (1) Preparation of surface-aminated silica nanoparticles with specificity for binding intracellular proteins: first suspend the surface-aminated silica nanoparticles of 100-120 nm in a glutaraldehyde solution with a mass fraction of 25%, Make the final concentration 1mg / ml, stir overnight at room temperature to activate the particles; wash 3 times with 3ml PBS (pH 8.0) the next day to remove excess glutaraldehyde solution; then resuspend in PBS (pH 8.0) to form 2.5mg / ml nanoparticle suspension;
[0060] Take 100 μl of anti-Akt2 antibody solution (purchased from Santa Cruz Biotechnology, derived from mice, antibody:PBS volume ratio = 1:800) and add it to the above...
Embodiment 3
[0068] Example 3: The expression of phosphorylated protein kinase B (p-Akt1 / 2 / 3) in the human cervical cancer Hela cell line is illustrated as an example.
[0069] 1. the preparation of silicon dioxide nanoparticle (same as embodiment 1)
[0070] 2. Preparation of nanoparticles with specificity for binding intracellular proteins
[0071] (1) Preparation of surface-aminated silica nanoparticles with specificity for binding intracellular proteins: first suspend the surface-aminated silica nanoparticles of 100-120 nm in a glutaraldehyde solution with a mass fraction of 25%, Make the final concentration 1mg / ml, stir overnight at room temperature to activate the particles; wash 3 times with 3ml PBS (pH 8.0) the next day to remove excess glutaraldehyde solution; then resuspend in PBS (pH 8.0) to form 2.5mg / ml nanoparticle suspension;
[0072] Add 100 μl of anti-p-Akt1 / 2 / 3 antibody solution (purchased from Santa Cruz Biotechnology, derived from mice, antibody: PBS volume ratio = 1:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com