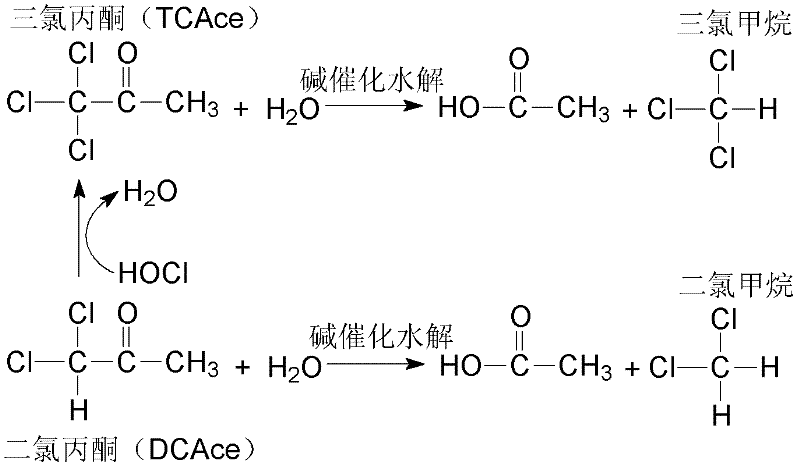
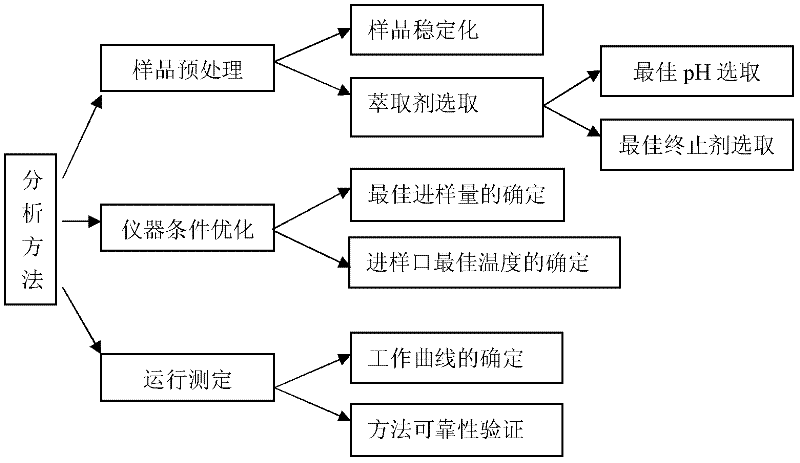
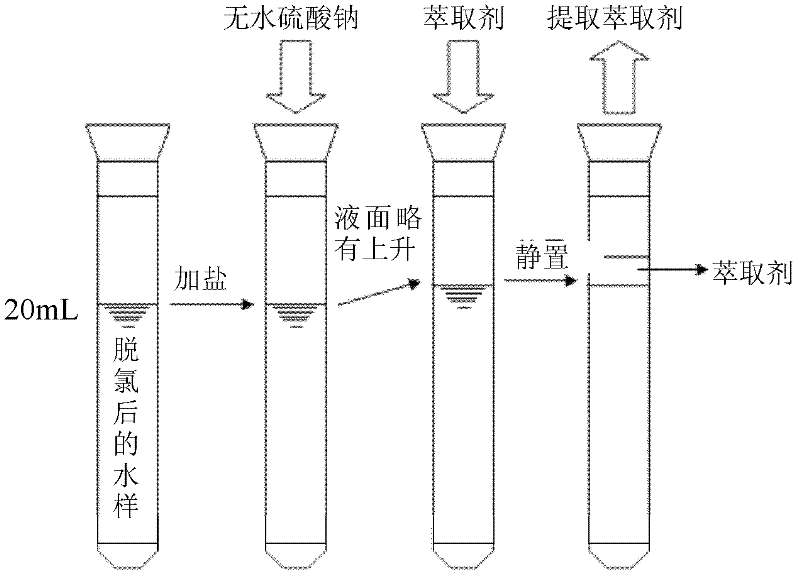
Method for accurately quantifying 1,1-dichloroacetone and 1,1,1-trichloroacetone in water
A technology of dichloroacetone and trichloroacetone, applied in the field of environmental engineering, achieves the effects of optimizing instrument conditions, avoiding interference, and increasing sample cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] figure 2 Represent the schematic flow sheet of the inventive method, below in conjunction with figure 2 Be explained.
[0032] 1 Sample pretreatment
[0033] 1.1 Selection of extractant
[0034] Firstly, the tap water samples were pretreated (see "1.2 DCAce and TCAce Stabilization Pretreatment", and then 4g of anhydrous sodium sulfate was added to the test tube containing 20mL of pretreated water samples, and manually shaken for 1min, so that no Sodium sulfate in water is fully dissolved, and the liquid level of the water sample rises. Then add 2mL of a certain extractant (examine different extractants, including n-pentane, n-hexane, methyl tert-butyl ether and ethyl acetate). The middle liquid level rises slightly, shake vigorously by hand for 5 minutes, let it stand for 10 minutes, extract the upper extractant solution, and perform GC / MS determination. image 3 Schematic representation of the extraction procedure. Through this experiment, it is found that compare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com