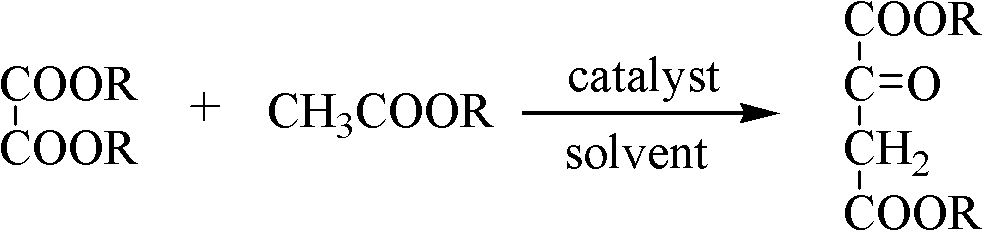Preparation method of medicine intermediate butanone diacid diester compound
A technology of isobutanedate diesters and compounds, which is applied in the field of preparation of pharmaceutical intermediates butanedate diesters, can solve the problems of unsuitability for industrial production, harsh reaction conditions, and low yield, and achieve catalyst Cheap and easy to obtain, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a reactor equipped with freezing, stirring, and a thermometer, 150ml of furan, 11.8g (0.1mol) of dimethyl oxalate, and 5.4g (0.1mol) of sodium methoxide were respectively added. Dissolve 8.2g (0.11mol) of methyl acetate in 50ml of furan, control the temperature in an ice-water bath at 5°C, add the methyl acetate / furan mixture dropwise to the dimethyl oxalate / furan mixture with stirring, within 2 hours After the dropwise addition, react at 5°C for another 2h, remove the ice-water bath, install a reflux condenser in the reactor, slowly raise the temperature to 30°C, and react for 6h. Wash with dilute hydrochloric acid to acidify the reaction solution to weak acidity, separate the liquids, wash twice with saturated sodium chloride solution, separate the liquids, dry over anhydrous sodium sulfate, filter, and recover furan by distillation under reduced pressure to obtain 8.7 g of butanone diacid Dimethyl ester, yield 52.2%, content (GC) 95.9%.
Embodiment 2
[0024] In a reactor equipped with freezing, stirring, and a thermometer, 150 ml of tetrahydrofuran, 11.8 g (0.1 mol) of dimethyl oxalate, and 2.7 g (0.05 mol) of sodium methoxide were respectively added. Dissolve 7.4g (0.1mol) of methyl acetate in 50ml of tetrahydrofuran, control the temperature in an ice-water bath at 5°C, and add the methyl acetate / tetrahydrofuran mixture dropwise to the dimethyl oxalate / tetrahydrofuran mixture with stirring, within 2 hours After the dropwise addition, react at 5°C for another 4h, remove the ice-water bath, install a reflux condenser in the reactor, slowly raise the temperature to 40°C, and react for 4h. Wash with dilute hydrochloric acid to acidify the reaction solution to weak acidity, separate the liquids, wash twice with saturated sodium chloride solution, separate the liquids, dry over anhydrous sodium sulfate, filter, and distill under reduced pressure to recover tetrahydrofuran to obtain 14.4 g of butanone diacid Dimethyl ester, yield...
Embodiment 3
[0026] In a reactor equipped with refrigeration, stirring, and a thermometer, 150ml of 2-methyltetrahydrofuran, 11.8g (0.1mol) of dimethyl oxalate, and 5.4g (0.1mol) of sodium methoxide were respectively added. Dissolve 11.1g (0.15mol) of methyl acetate in 100ml of 2-methyltetrahydrofuran, control the temperature in an ice-water bath at 0°C, and add the mixture of methyl acetate / 2-methyltetrahydrofuran dropwise to dimethyl oxalate under stirring / 2-Methyltetrahydrofuran mixture, the dropwise addition was completed within 2 hours, reacted at 0°C for another 2h, removed the ice-water bath, installed a reflux condenser for the reactor, slowly raised the temperature to 60°C, and reacted for 2h. Wash with dilute hydrochloric acid to acidify the reaction solution to weak acidity, separate the liquids, wash twice with saturated sodium chloride solution, separate the liquids, dry over anhydrous sodium sulfate, filter, and recover 2-methyltetrahydrofuran by distillation under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com