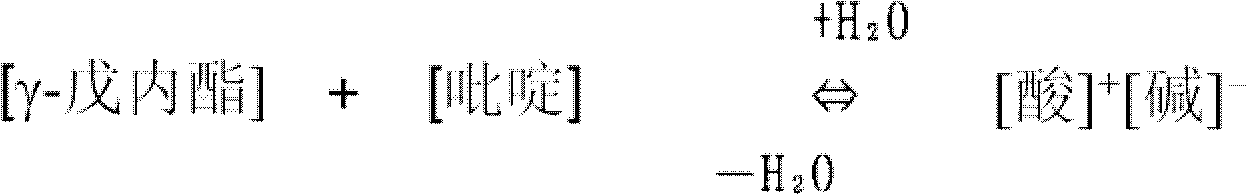
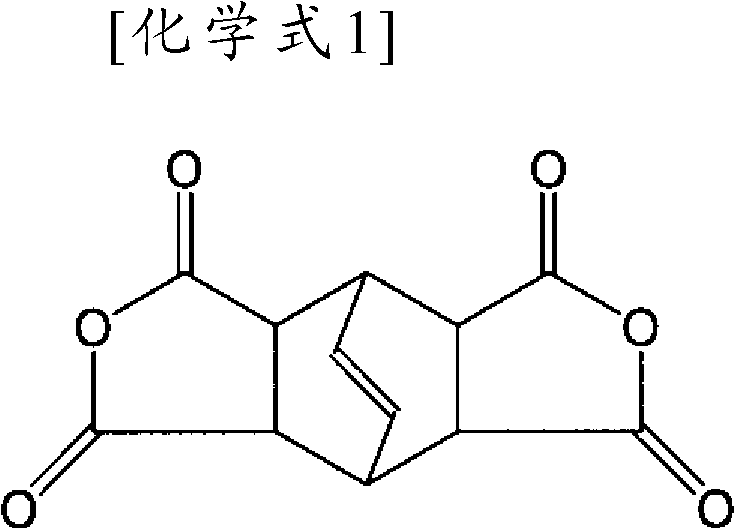
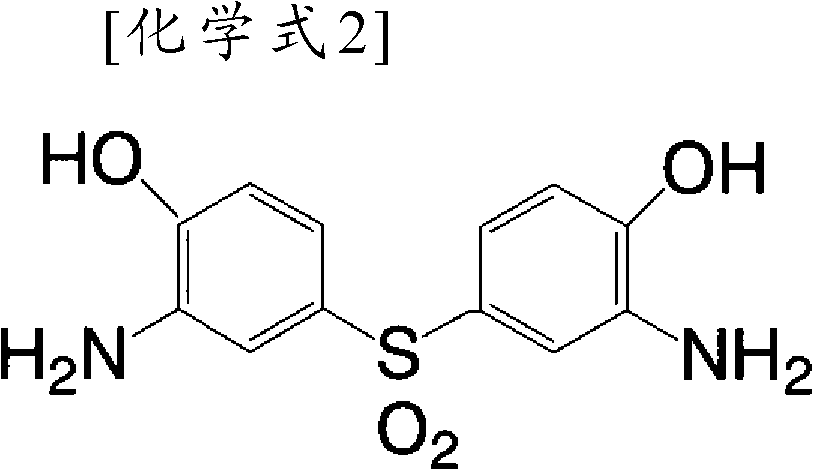
Organic-solvent-soluble polyimide comprising PMDA, DADE, BPDA, and BCD
A technology of polyimide and organic solvent, applied in the field of bicyclooctene tetracarboxylic dianhydride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
corresponding Embodiment 1
[0090] Corresponding to the manufacturing method of embodiment 1
[0091] Contains the following three stages:
[0092] (a) In the first stage, 2 molar equivalents of pyromellitic dianhydride (PMDA) and bis(3-amino-4-hydroxyphenyl)sulfone (HOAB·SO 2 ) 1 molar equivalent reacts at 160~200 DEG C in the presence of a catalyst in a polar organic solvent to generate an oligomer whose two ends are PMDA,
[0093] (b) In the second stage, the oligomer generated in the first stage reacts with 2 molar equivalents of bicyclooctene tetracarboxylic dianhydride (BCD) and 4 molar equivalents of 3,4'-diaminodiphenyl ether (mDADE) , generating oligomers with mDADE at both ends, and
[0094] (c) In the third stage, add 2 molar equivalents of biphenyltetracarboxylic dianhydride (BPDA) and 1 molar equivalent of 1,3-bis(4-aminophenoxy)benzene (mTPE), react, polycondensate, and synthesize Polyimide copolymer soluble in polar organic solvents.
corresponding Embodiment 2
[0095] Corresponding to the manufacturing method of embodiment 2
[0096] Contains the following three stages:
[0097] (a) In the first stage, 2 molar equivalents of pyromellitic dianhydride (PMDA) and bis(3-amino-4-hydroxyphenyl)sulfone (HOAB·SO 2 ) 1 molar equivalent reacts at 160~200 DEG C in the presence of a catalyst in a polar organic solvent to generate an oligomer whose two ends are PMDA,
[0098] (b) In the second stage, the oligomer generated in the first stage reacts with 2 molar equivalents of bicyclooctene tetracarboxylic dianhydride (BCD) and 4 molar equivalents of 3,4'-diaminodiphenyl ether (mDADE) , generating oligomers with mDADE at both ends, and
[0099] (c) In the third stage, add 2 molar equivalents of benzophenone tetracarboxylic dianhydride (BTDA) and 1 molar equivalent of 1,3-bis(4-aminophenoxy)benzene (mTPE), react and polycondense, Synthesis of polyimide copolymers soluble in polar organic solvents.
[0100] In the reaction of embodiment 1 and ...
Embodiment 1
[0106] The composition ratio of embodiment 1 is (PMDA) 2 (mDADE) 4 (BCD) 2 (HOAB SO 2 ) 1 (mTPE) 1 (BPDA) 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com