Prodrugs of guanfacine
一种胍法辛、前药的技术,应用在胍法辛的各种前药领域,能够解决药物暴露的速率和程度差异性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
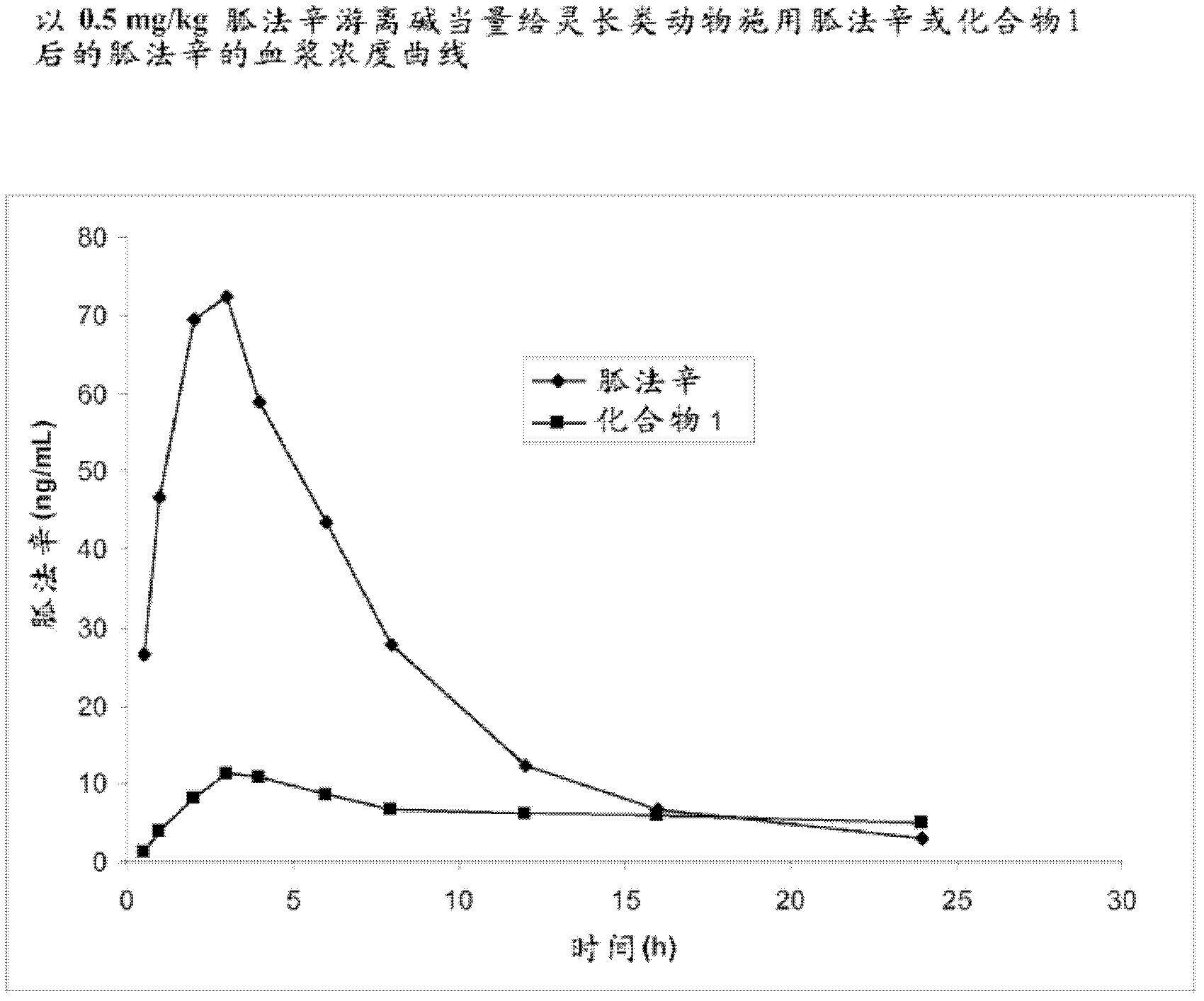
[0296] Example 1. Preparation of Guanfacine-Glutaryl-Valine Amide (Compound 1)
[0297] The synthesis of guanfacine-[glutaryl-(S)-valine]amide trifluoroacetate was completed in four steps. Glutaryl-(S)-valine tert-butyl ester was obtained by reacting (S)-valine tert-butyl ester with glutaric anhydride. The 'activated ester' was prepared from glutaryl-(S)-valine tert-butyl ester by DCC coupling with N-hydroxysuccinimide. This ester is then reacted with guanfacine to give guanfacine-[glutaryl-(S)-valine]amide tert-butyl ester. Removal of the tert-butyl group was accomplished by treatment with trifluoroacetic acid to give guanfacine-[glutaryl-(S)-valine]amide trifluoroacetate. The synthetic route is shown in Scheme 1 below.
[0298] Process 1:
[0299]
[0300] LCMS: m / z = 457.00 for the deprotonated ion (M-H) - is consistent
[0301] of
[0302] 1 H NMR (DMSO-d 6): 9.72 (br s, 3H, 3×NH), 8.00 (d, J=8.5Hz, 1H,
[0303] NH), 7.51(d, J=7.8Hz, 2H,...
Embodiment 2
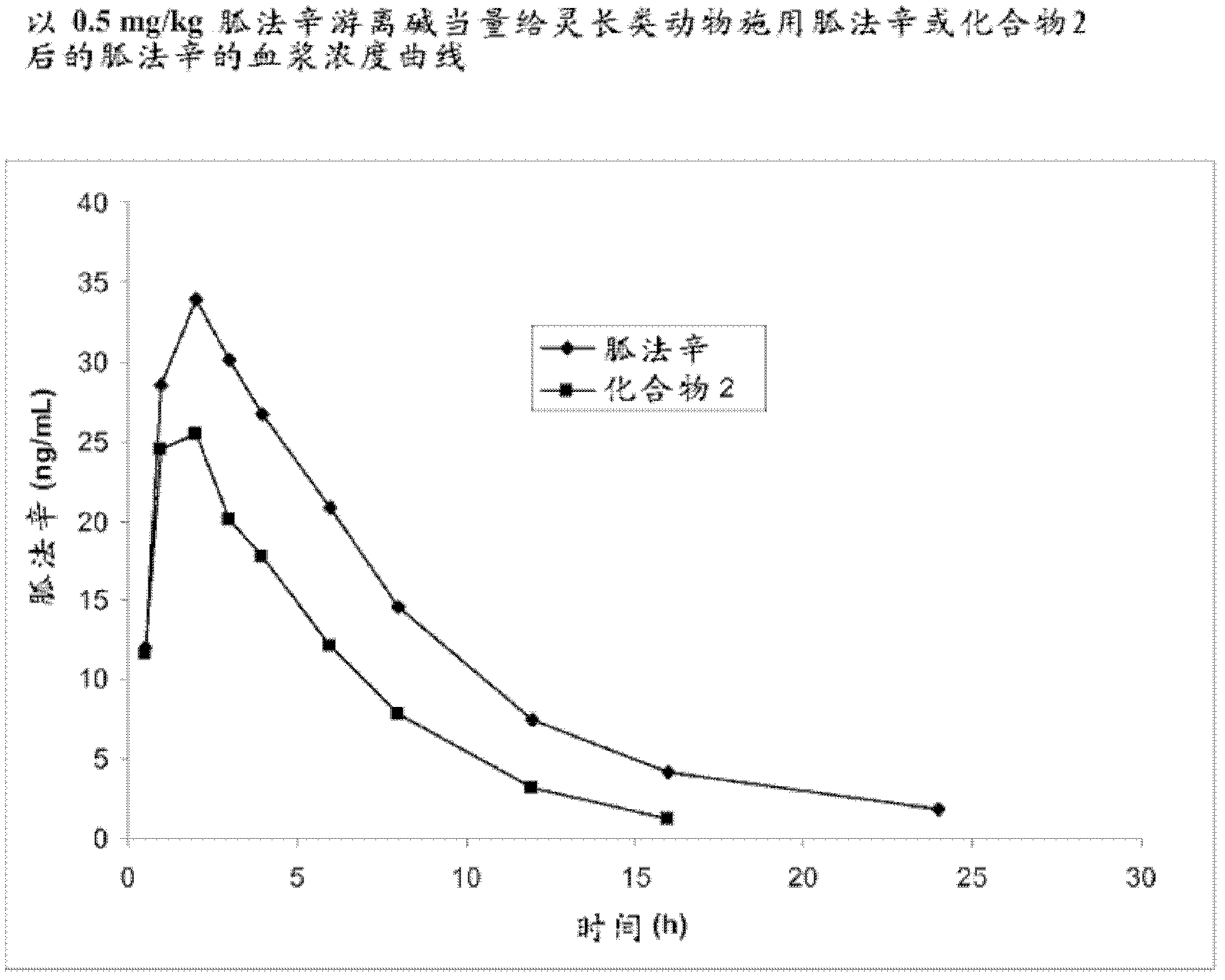
[0308] Example 2. Synthesis of Guanfacine-β-Alanine-Valine Amide (Compound 2)
[0309] The synthesis of guanfacine-β-alanine-(S)-valine amide di-trifluoroacetate was accomplished in six steps. N-Boc-(S)-valine was treated with DCC and N-hydroxysuccinimide to give the first 'activated ester', which was then coupled with β-alanine benzyl ester. Subsequent debenzylation affords N-Boc-(S)-valine-β-alanine, which is then converted to a second 'activated ester' via DCC coupling with N-hydroxysuccinimide. The activated ester is coupled with guanfacine to give N-Boc-(S)-valine-β-alanine-guanfacine. Removal of the Boc protecting group was accomplished by treatment with trifluoroacetic acid to give guanfacine-β-alanine-(S)-valine amide di-trifluoroacetate. The synthetic route is shown in Scheme 2 below.
[0310] Process 2:
[0311]
[0312] LCMS: m / z = 414.00 for the deprotonated ion (M-H) - is consistent
[0313] 1 H 9.67 (br, 2H, NH 2 ), 8.52 (m, 1 H, NH), 8.10 (br, 3 H,
...
Embodiment 3
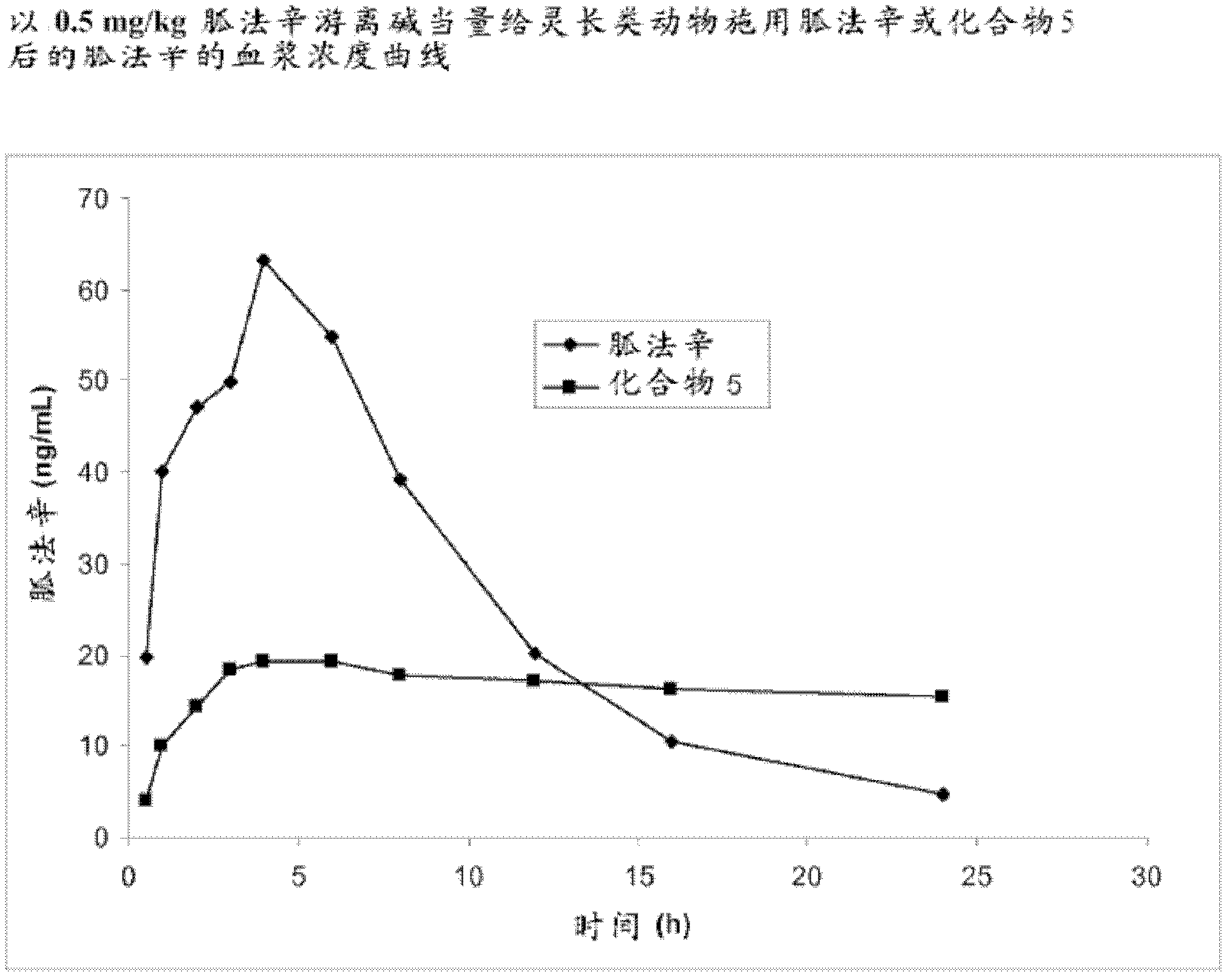
[0319] Example 3. Synthesis of Guanfacine-γ-Glutamyl-(R)-Valine Amide (Compound 5)
[0320] The synthesis of guanfacine-γ-(S)-glutamate-(R)-valineamide di-trifluoroacetate was accomplished by a method involving six reaction steps. N-Boc-(R)-valine is first treated with DCC and N-hydroxysuccinimide to obtain the first 'activated ester'. This 'activated ester' is then coupled with H-Glu(OBn)-OtBu followed by debenzylation to give N-Boc-(R)-valine-(S)-glutamic acid tert-butyl ester.
[0321] It is converted to a second 'activated ester' by DCC coupling with N-hydroxysuccinimide and this ester is reacted with guanfacine to give N-Boc-(R)-valine-(S)-glutamic acid (Guanfacine) tert-butyl ester. Removal of the tert-butyl ester and the Boc protecting group was successfully achieved using trifluoroacetic acid to give guanfacine-γ-(S)-glutamate-(R)-valine amide di-trifluoroacetate. The synthetic route is shown in Scheme 3.
[0322] Process 3:
[0323]
[0324] LCMS: m / z = 473.96...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com