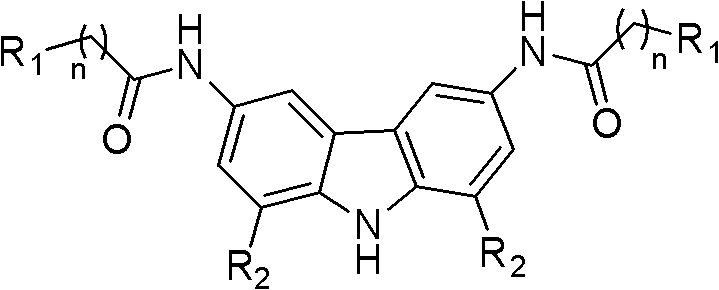
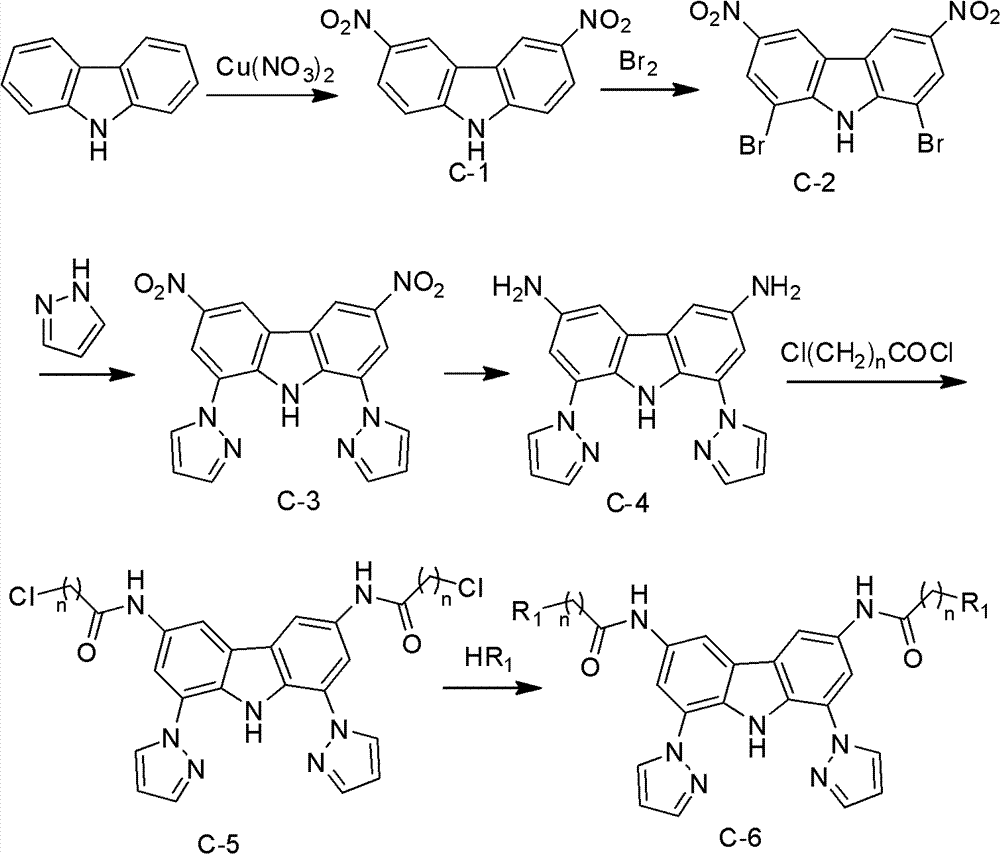
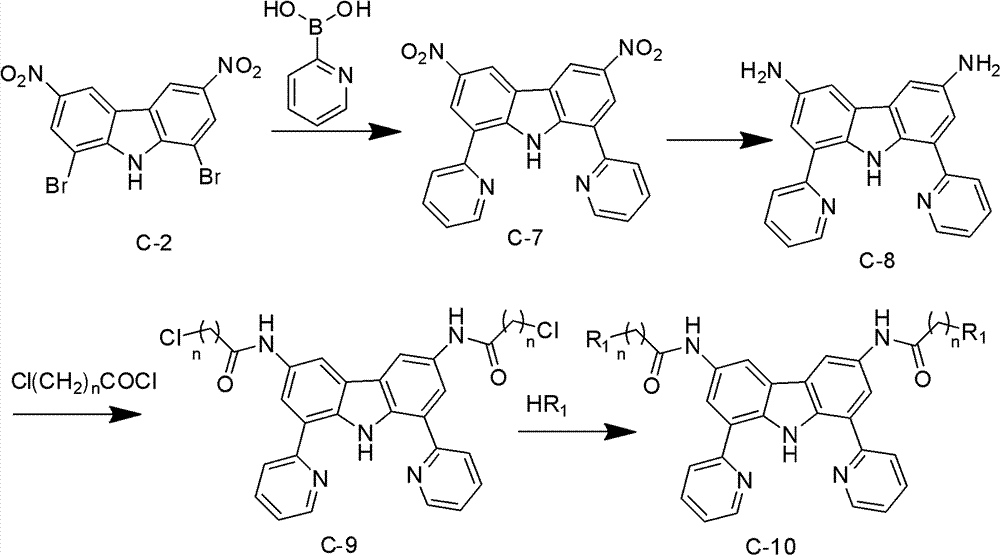
Carbazole derivative, preparation method thereof, and application of carbazole derivative serving as anticancer drug
A technology of carbazole derivatives and compounds, which is applied in the field of medicine and chemical industry, can solve the problems of less development and research of carbazole derivatives, and achieve the effects of low normal cell toxicity, good inhibitory activity, and tumor inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment one: the synthesis of compound C-1
[0031] Dissolve 35g of copper nitrate in a mixture of 96ml of acetic acid and 145ml of acetic anhydride, slowly add 20g of carbazole over 10min under an ice bath, and stir at room temperature for 0.5 hours. Then react at 90° C. for 0.5 h, cool down, pour the reaction solution into water, stir continuously, filter with suction, and wash with water to obtain a yellow solid. The filter cake was added to cold 100gKOH and 1500ml ethanol: water 1:1 mixed solution, stirred for 0.5h, filtered, the filtrate was acidified with concentrated hydrochloric acid, and a yellow solid was precipitated, filtered with suction, washed with water, dried, and subjected to silica gel column chromatography (petroleum enzyme / ethyl acetate), to obtain pale yellow solid powder C-1.
[0032] Yield: 70%; 1 H NMR (400MHz, DMSO) δ12.69(s, 1H), 9.50(d, J=2.1Hz, 1H), 8.40(dd, J=9.0, 2.1Hz, 1H), 7.77(d, J=9.0Hz , 1H); ESI-MS m / z: 258[M+H] + .
[0033]...
Embodiment 2
[0035] Embodiment two: the synthesis of compound C-2
[0036] Dissolve 2gC-1 in 30ml concentrated sulfuric acid, then raise the temperature to 90°C, slowly add bromine into the reaction solution, react for 2h, stop the reaction, cool, pour into water, filter, wait for yellow solid, silica gel column chromatography (petroleum ether / acetone) to give C-2 as a pale yellow solid.
[0037] Yield: 50%; 1H NMR (400MHz, DMSO) δ12.72(s, 1H), 9.49(d, J=2.1Hz, 2H), 8.54(d, J=2.1Hz, 2H); ESI-MS m / z:416[M+H] + .
[0038]
[0039] Compound C-2
Embodiment 3
[0040] Embodiment three: the synthesis of compound C-3
[0041] Dissolve 2g of compound C-2 in 30ml of NMP, add 1g of pyrazole, 100mg of Cu 2 O, 200°C, reacted for 24h, cooled, poured the reaction solution into water, stirred continuously, precipitated solid, filtered to obtain a yellow solid, alumina column chromatography (dichloromethane) to obtain a yellow solid C-3.
[0042] Yield: 35%; H NMR (400MHz, DMSO) δ12.83(s, 1H), 9.35(d, J=1.8Hz, 2H), 9.02(d, J=2.6Hz, 2H), 8.76(d, J=1.9Hz, 2H), 8.01(d, J=1.7Hz, 2H), 6.72-6.74(m, 2H).ESI-MS m / z: 390[M+H] + .
[0043]
[0044] Compound C-3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com