Strains of s.cerevisiae, capable of growing in media with melibiose, stachyose and raffinose
A yeast and genetic technology, applied in the field of Saccharomyces cerevisiae strains, can solve problems such as changing DNA, low growth rate, interfering with replication and transcription process growth and respiration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Obtaining a modified yeast strain for the production of α-galactosidase
[0091] Materials and methods
[0092] 1. Cloning of the nucleotide sequence from the gene encoding α-galactosidase
[0093]Two pairs of primers were designed to amplify the MEL1 gene encoding Saccharomyces cerevisiae α-galactosidase by PCR (Polymerase Chain Reaction, polymerase chain reaction). Two fragments are amplified, one of which corresponds to the complete gene of α-galactosidase (SEQ ID NO: 4), using the following primers with SEQ ID NO: 5 and SEQ ID NO: 6 for amplification; the other fragment corresponds to the removal of the coding secretion The 54-nucleotide α-galactosidase gene of the signal (SEQ ID NO: 7) was amplified by the following primers having SEQ ID NO: 8 and SEQ ID NO: 9. Insert the complete gene of α-galactosidase into the vector YEpFLAG1 (Eastman Kodak classification number IB13400) for ADH2 (Alcohol Dehydrogenase, alcohol dehydrogenase 2) promoter (sequence number: 1...
Embodiment 2
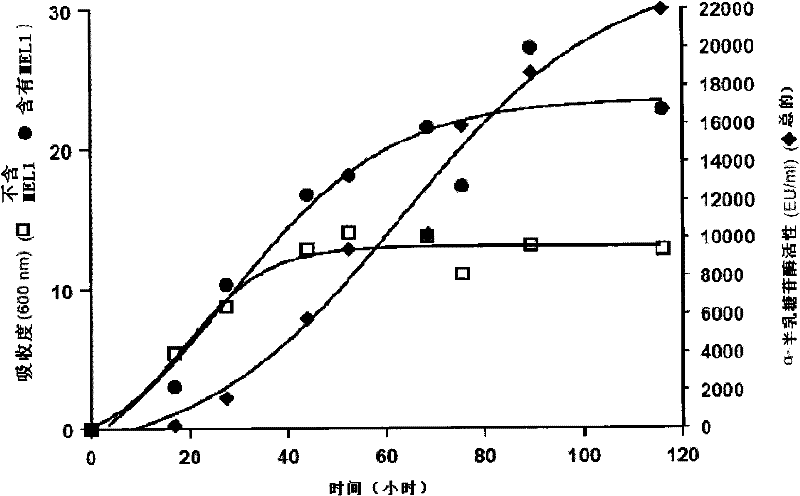
[0107] Strains [A] with MEL1 under the ADH2 promoter and strains with MEL1 under the ADH1 promoter Comparison of α-galactosidase activity of strain [B]
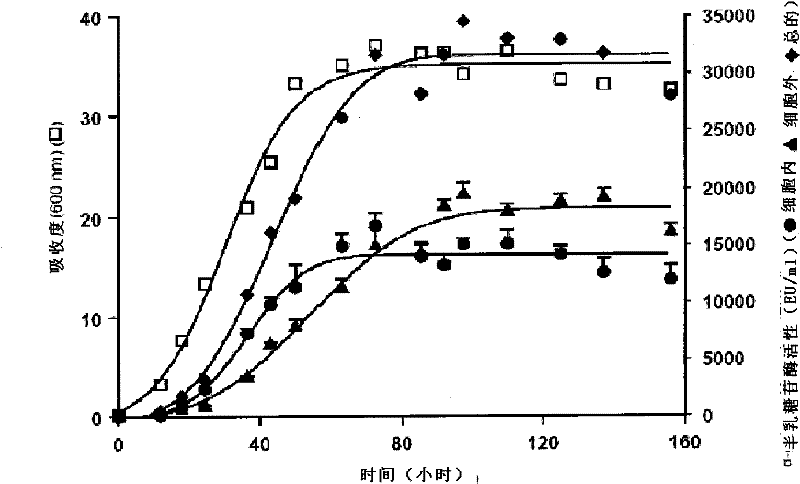
[0108] The details of cloning and the determination of α-galactosidase activity are described in Example 1. The recombinant strain [A] was cultured on a synthetic medium containing 1% glucose.
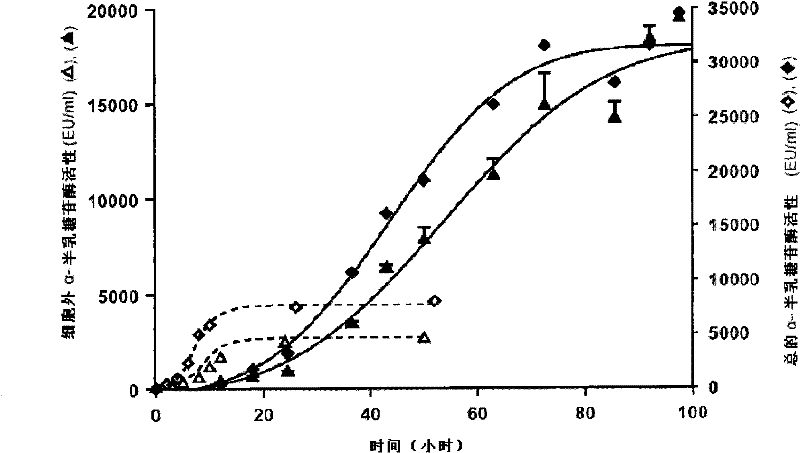
[0109] figure 2 Shows the comparison of the data obtained from the strain [B] described in US Patent No. 5,055,401 and the recombinant strain [A] of the present invention. For purposes, data were extracted from Figures 7A and 7B in US 5055401 and they have been compared. It can be observed that the total activity of the α-galactosidase of the strain described in US 5055401 reaches 8000E.U. / ml in 36-54 hours, while the total activity of the strain [A] of the present invention reaches the total The activity was 10,000 E.U. / ml to 20,000 E.U. / ml and, as discussed previously, reached about 32,000 E.U. / ml at a later stage of the cul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com