Method for purifying detoxified pertussis vaccine antigen solution
A vaccine antigen and antigen solution technology, applied in the direction of recovery/purification, bacterial antigen components, antibacterial drugs, etc., can solve the problems of decreased work efficiency and harvest rate, protein precipitate accumulation and blockage, unsuitable for industrialization, etc., and achieve improvement Ultrasonic homogenization efficiency, slow sedimentation speed, and the effect of improving potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Main materials and equipment:
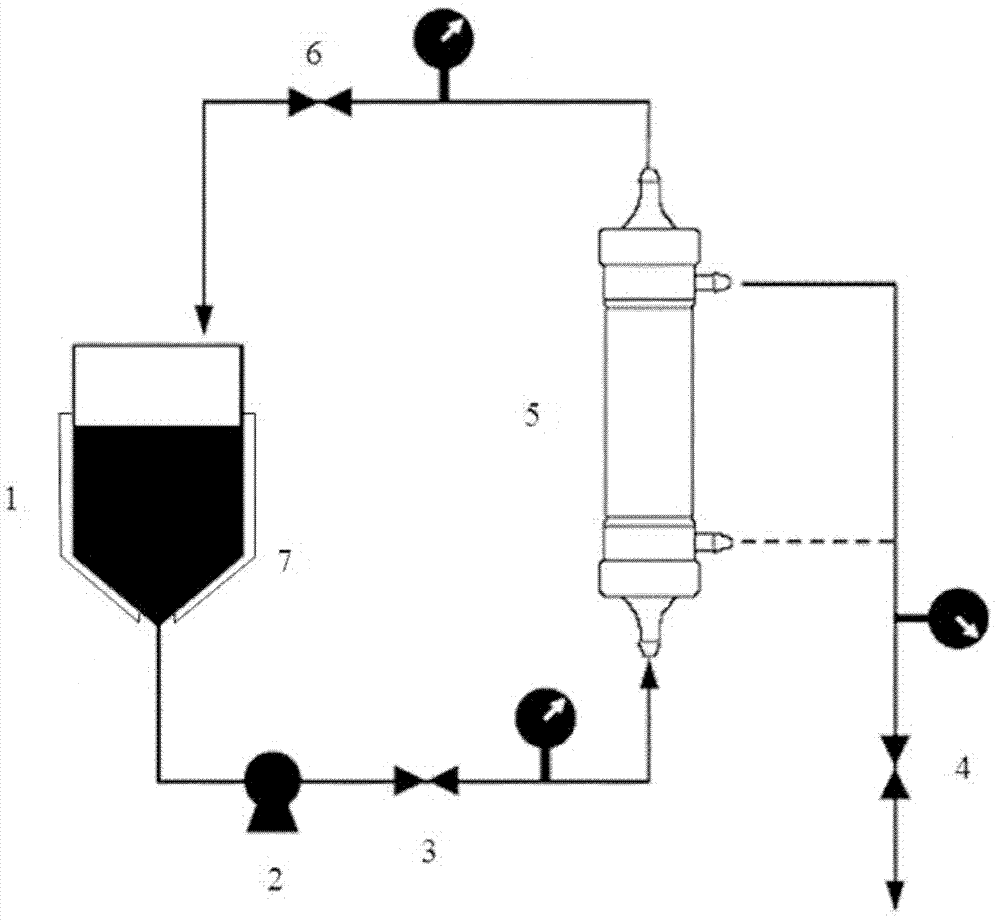
[0026] Wash filtrate (pH6.5-7.5, sodium phosphate buffer 5 millimoles per liter, sodium chloride 0.5 moles per liter); 6000 milliliters of refined acellular pertussis antigen solution after detoxification (protein content is 75 micrograms of protein nitrogen per milliliter) , hereinafter referred to as the detoxified antigen solution); 10kDa hollow fiber ultrafiltration column: membrane column model, SLP-1053 (PALL company); liquid supply pump Quottroflow sanitary grade diaphragm pump, filter valve is a band flow meter (Rosemount 8731 Type sanitary flowmeter) diaphragm valve; high-speed refrigerated centrifuge (Eppendorf), small vertical electrophoresis instrument (Bio-rad), electronic balance (Mettler).
[0027] Specific process:
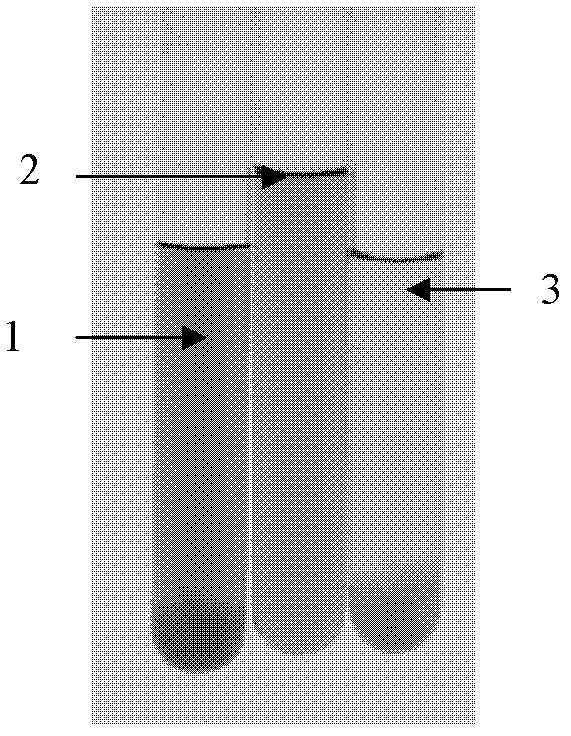
[0028] Step 1, pre-adjustment: adjust the concentration of sodium chloride in the detoxified refined acellular pertussis antigen solution to 0.5 moles per liter, the conductivity is 40ms / cm, and prepare the w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com