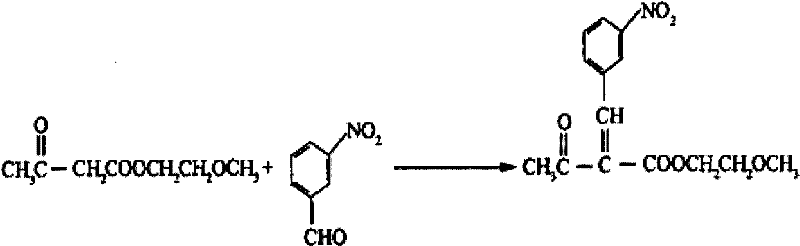
Synthesis method of methoxy ethyl 2-(3-nitrobenzylidene)acetacetate
A technology of methoxyethyl acetate and acetoacetate methoxyethyl, applied in the field of synthesis of cilnidipine condensation intermediate 2-acetoacetate methoxyethyl, can solve the problem of low yield, poor purity, etc. problems, to achieve the effect of reducing side reactions, shortening the reaction time, and changing the feeding method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Weigh 200g of methoxyethyl acetoacetate, add it to a 500ml three-neck flask, stir, cool down to about 5°C, add 25ml of concentrated sulfuric acid dropwise, keep the temperature below 10°C, and complete the dropwise addition in 40 minutes. Ethyl acetate 100ml, then add m-nitrobenzaldehyde 151g in three equal parts;
[0025] (2) React at room temperature for 2.5 hours, stop stirring, and place overnight;
[0026] (3) Add 450ml of ethyl acetate the next day to dissolve all the solids, add 200ml of purified water to extract twice, and collect the organic layer;
[0027] (4) Ethyl acetate is evaporated under reduced pressure, cooled with ice water, and crystallized;
[0028] (5) The obtained crystals were filtered and washed with a small amount of ethanol to obtain a light yellow solid, which was dried at 70° C. to obtain 208 g of a solid, with a yield of 71% and a content of 99.2% (analyzed by HPLC).
Embodiment 2
[0030] (1) Weigh 164g of methoxyethyl acetoacetate, add it to a 500ml three-neck flask, stir, cool down to about 5°C, add 23ml of concentrated sulfuric acid dropwise, keep the temperature below 10°C, and complete the dropwise addition in 30 minutes. Ethyl acetate 105ml, and then add m-nitrobenzaldehyde 130g in five equal parts;
[0031] (2) React at room temperature for 2.5 hours, stop stirring, and place overnight;
[0032] (3) The next day, add 350ml of ethyl acetate to dissolve all the solids, add 160ml of purified water to extract twice, and collect the organic layer;
[0033] (4) Ethyl acetate is evaporated under reduced pressure, cooled with ice water, and crystallized;
[0034] (5) The obtained crystals were filtered and washed with a small amount of ethanol to obtain a light yellow solid, which was dried at 70° C. to obtain 184 g of a solid, with a yield of 73% and a content of 99.0% (analyzed by high performance liquid chromatography).
Embodiment 3
[0036] (1) Weigh 279g of methoxyethyl acetoacetate, add it to a 1000ml three-neck flask, stir, cool down to about 5°C, add 35ml of concentrated sulfuric acid dropwise, keep the temperature below 10°C, and complete the dropwise addition in 50 minutes. Ethyl acetate 200ml, and then add m-nitrobenzaldehyde 220g in five equal parts;
[0037] (2) React at room temperature for 3.0 hours, stop stirring, and place overnight;
[0038] (3) The next day, add 600ml of ethyl acetate to dissolve all the solids, add 280ml of purified water to extract three times, and collect the organic layer;
[0039] (4) Ethyl acetate is evaporated under reduced pressure, cooled with ice water, and crystallized;
[0040] (5) The obtained crystals were filtered and washed with a small amount of ethanol to obtain a light yellow solid, which was dried at 70° C. to obtain 321 g of a solid with a yield of 75% and a content of 99.1% (analyzed by HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com