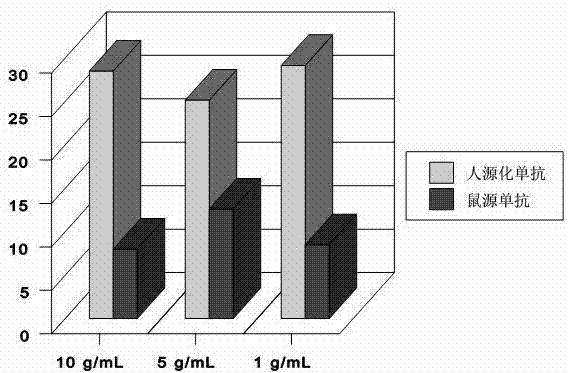
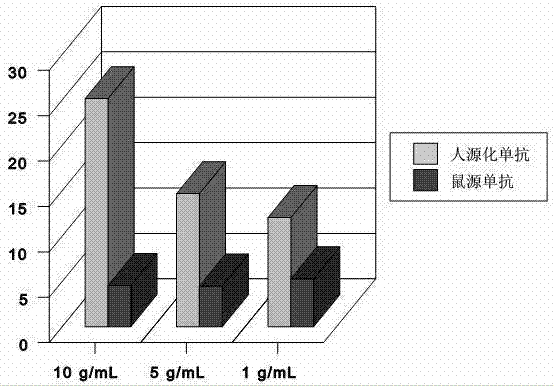
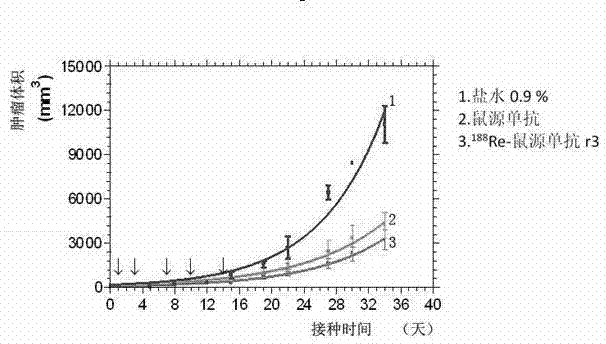
Application of monoclonal antibody in treating esophageal cancer
A monoclonal antibody, esophageal cancer technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve problems such as poor efficacy of esophageal cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Pre-clinical pharmacodynamic test
[0035] 1. In vitro activity
[0036] Nimotuzumab and H-125 human lung adenocarcinoma tumor cell line, A431 human vaginal epithelial cancer tumor cell line, and MDA-MB-468 human breast cancer tumor cell line were tested by the balanced binding test of 99mTc labeled Nimotuzumab The ability of monoclonal antibodies to bind to tumor cells. Labeled using a freeze-drying kit (purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd.) at room temperature. After 15 minutes, an average of (97.38±0.23)% of 99mTc was labeled on the IgG antibody. Fast paper chromatography of the monoclonal antibody in acetone showed that about 3% or less of free pertechnetic acid ran to the front of the reagent (Rf=10). This reveals that almost all pertechnetic acid has been reduced. The colloidal morphology of Nimotuzumab measured by the ITLC method impregnated with 1.0% albumin was (1.68±0.81)%. The maximum binding (Bmax) of 99mTc-labeled nimotuzumab ...
Embodiment 2
[0055] Example 2. Safety evaluation of clinical anti-tumor:
[0056] One observes the tolerability and efficacy of radiotherapy combined with nimotuzumab combined with radiotherapy in the treatment of esophageal cancer. A total of 42 patients with stage II esophageal cancer (squamous cell carcinoma) who were inoperable or refused surgery in stage II and only metastasized to the supraclavicular lymph nodes received conformal radiotherapy (DT50-70Gy), and were given 200 mg nitoplasty weekly at the same time Belizumab treatment. In 37 patients, Taixinsheng used more than 5 times (88.1%). It was found that 1-2 grade toxic side effects (21.4%) mainly included 1 case of digestive tract reaction, 3 cases of esophagitis, 4 cases of skin reaction, and 1 case of bone marrow suppression. Fever occurred in 9 patients, 5 cases of grade 1 lung injury, 3 cases of lung infection, 2 cases of fatigue, other paresthesias, sepsis, herpes zoster, increased bilirubin, heart discomfort, and esophagot...
Embodiment 3
[0058] Example 3. Toxicity test
[0059] 1. Acute Toxicology Research
[0060] The toxicity test of single-dose injection was performed on Sprague Dawley rats in accordance with the requirements of GLP standards. The doses are respectively 0, 0.25, 1.25 and 5 times the highest human dose of 400 mg (5.71 / mg / Kg). Each of the four groups of animals has 5 female and 5 male rats. Except for the increase in the number of soft stools in all animals (p<0.05), there were no deaths and clinical abnormalities in the administration and the control group. Conclusion: No obvious toxicity characteristics were found in any dose of the injection.
[0061] 2. Subacute toxicity study
[0062] A subacute study of repeated doses of nimotuzumab was done on animals (two rat trials and one monkey trial) with a dose of 4.0 to 12.7 times the highest human dose per kilogram (315mg / dose or 4.5mg / Kg). ), the monoclonal antibody is produced from the main cell bank and the expanded cell bank. The products prod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com