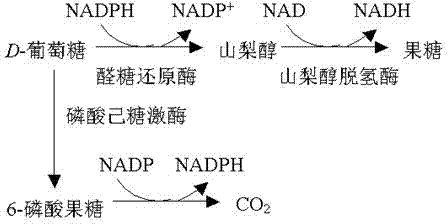
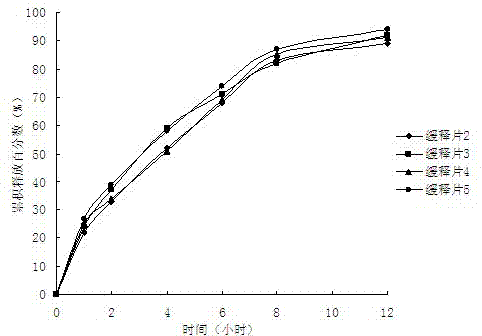
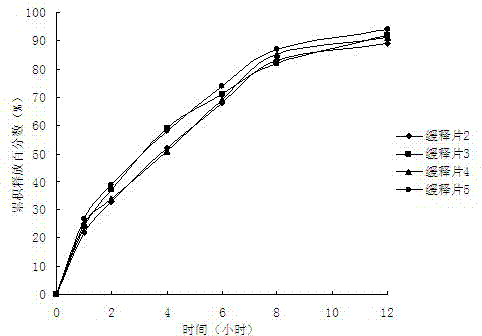
Epalrestat slow-release tablet and preparation method thereof
A technology of epalrestat and sustained-release tablets, which is applied in the field of sustained-release preparations and its preparation, can solve the problems of short half-life and poor patient compliance, and achieve the effects of convenient taking, easy operation, good clinical significance and commercial significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Epalrestat Sustained Release Tablet 1 (25mg / tablet, 1000 tablets in total)
[0041] Epalrestat 25g
[0042] Hydroxypropyl methylcellulose (K4M) 50g
[0043] Hydroxypropyl methylcellulose (K100) 20g
[0044] Lactose 652g
[0046] Preparation method: crush epalrestat, hydroxypropyl methylcellulose (K4M), hydroxypropyl methylcellulose (K100), lactose, pass through a 100-mesh sieve, mix mechanically, and add 3% polyvinyl chloride into the stirring An appropriate amount of 95% ethanol solution of ketone (K30), granulated, dried, and granulated. Add magnesium stearate to the above-mentioned dry granules after granulation, place in a granulator and mix evenly, control the hardness of the tablet at 7-8Kg, and tablet to obtain. A total of 1000 tablets were produced.
[0047]
Embodiment 2
[0049] Preparation of Epalrestat Sustained Release Tablet 1 (50mg / tablet, 1000 tablets in total)
[0050] Epalrestat 50g
[0051] Hydroxypropyl methylcellulose (K4M) 50g
[0052] Hydroxypropyl methylcellulose (K100) 50g
[0053] Lactose 547g
[0055] Preparation method: crush epalrestat, hydroxypropyl methylcellulose (K4M), hydroxypropyl methylcellulose (K100), lactose, pass through a 100-mesh sieve, mix mechanically, and add 3% polyvinyl chloride into the stirring An appropriate amount of 95% ethanol solution of ketone (K30), granulated, dried, and granulated. Add magnesium stearate to the above-mentioned dry granules after granulation, place in a granulator and mix evenly, control the hardness of the tablet at 7-8Kg, and tablet to obtain. A total of 1000 tablets were produced.
[0056]
Embodiment 3
[0058]Preparation of Epalrestat Sustained Release Tablet 2 (75mg / tablet, 1000 tablets in total)
[0059] Epalrestat 75g
[0060] Hydroxypropyl methylcellulose (K4M) 50g
[0061] Ethyl cellulose (N20) 50g
[0062] Lactose 522g
[0064] Preparation method: Grind epalrestat, hydroxypropylmethylcellulose (K4M), ethylcellulose (N20), lactose, pass through a 100-mesh sieve, mix mechanically, add 3% povidone (K30 ) in an appropriate amount of 95% ethanol solution, granulated, dried, and granulated. Add magnesium stearate to the above-mentioned dry granules after granulation, place in a granulator and mix evenly, control the hardness of the tablet at 7-8Kg, and tablet to obtain. A total of 1000 tablets were produced.
[0065]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com