Method for preparing amino-terminated polyurethane
A technology of amino-terminated polyurethane and hydroxyl-terminated polyurethane, which is applied in the field of preparation of active amino-terminated functional compounds, and can solve problems such as poor fatigue resistance, heat resistance and impact resistance, affecting the performance of amino-terminated polyurethane, and difficulty in guaranteeing the performance of sprayed products, etc. , to achieve low cost, reduce production risk, and inhibit the generation of cross-linked structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
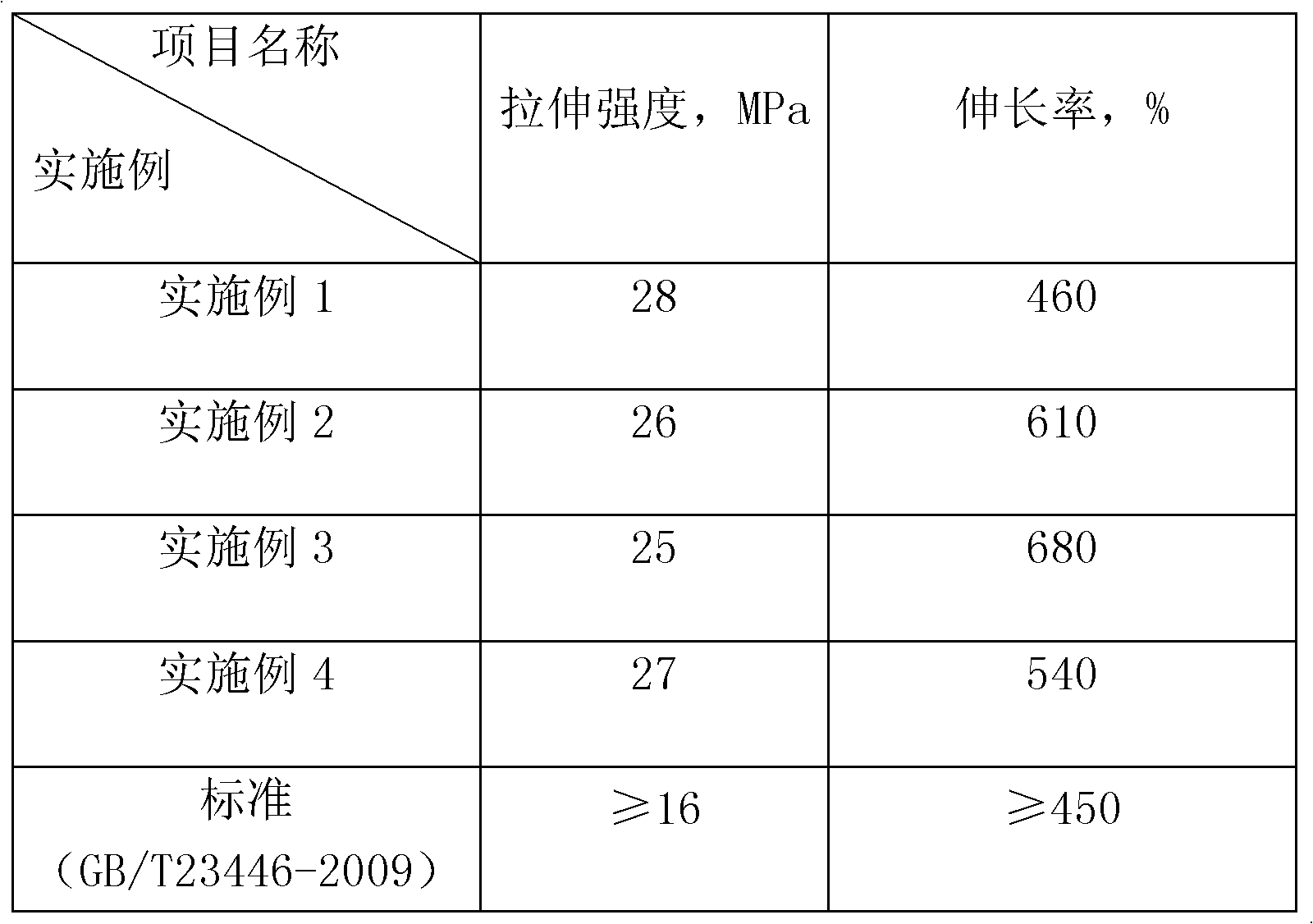
Embodiment 1
[0017] Step 1: Add 10 mol of propylene oxide homopolyether diol with a molecular weight of 400 into the reactor, vacuum dehydrate at 90-100°C for 1-2 hours, take samples to measure the moisture content, when the moisture content is less than 0.08%, Stop dehydration, cool to 70-75°C and maintain at this temperature range, add organic bismuth BiCAT catalyst, add 10mol MDI dropwise while stirring, continue to keep warm for 3 hours after the dropwise addition, and obtain hydroxyl-terminated polyurethane with an average molecular weight of 1050.
[0018] The second step: Slowly add the hydroxyl-terminated polyurethane obtained in the first step into another kettle equipped with 20mol IPDI and organic bismuth BiCAT catalyst under rapid stirring, and react for 3 hours to obtain an isocyanate-terminated polyurethane with an average molecular weight of 1495.
[0019] The third step: lower the temperature to below 30°C, under the condition of rapid stirring, slowly add the product obtain...
Embodiment 2
[0021] Step 1: Add 10 mol of ethylene oxide homopolyether diol with a molecular weight of 1000 into the reaction kettle, vacuum dehydrate at 90-100°C for 1-2 hours, take samples to measure the moisture content, when the moisture content is less than 0.08% , stop dehydration, cool to 70-75°C and maintain at this temperature range, add an organotin catalyst, add 10mol MDI dropwise while stirring, continue to keep warm for 3 hours after the dropwise addition, and obtain a hydroxyl-terminated polyurethane with an average molecular weight of 2250.
[0022] The second step: Slowly add the hydroxyl-terminated polyurethane obtained in the first step into another kettle equipped with 20 mol of IPDI and organic bismuth BiCAT catalyst under rapid stirring, and react for 3 hours to obtain an isocyanate-terminated polyurethane with an average molecular weight of 2695.
[0023] The third step: lower the temperature to below 30°C, under the condition of rapid stirring, slowly add the product ...
Embodiment 3
[0025] Step 1: Add 10 mol of ethylene oxide / propylene oxide copolyether diol with a molecular weight of 2000 into the reaction kettle, vacuum dehydrate at 90-100°C for 1-2 hours, take samples to measure the moisture content, when the moisture content is less than 0.08%, stop dehydration, cool to 70-75°C and maintain at this temperature range, add organotin and organobismuth BiCAT in a mass ratio of 1:1 composition, add 10mol HDI dropwise while stirring, continue to Insulated for 3 hours to obtain a hydroxyl-terminated polyurethane with an average molecular weight of 4250.
[0026] The second step: Slowly add the hydroxyl-terminated polyurethane obtained in the first step into another kettle equipped with 20 mol of IPDI and organic bismuth BiCAT catalyst under rapid stirring, and react for 3 hours to obtain an isocyanate-terminated polyurethane with an average molecular weight of 4695.
[0027] The third step: lower the temperature to below 30°C, under the condition of rapid st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com