Enzymatic resolution method for optical pure 2-amino-2'-hydroxy-1,1'-binaphthyl
An amino and hydroxyl technology, applied in the field of preparation of organic compounds, can solve the problems of high boiling point of anisole, difficult to remove, immature synthesis and splitting of NOBIN, etc., and achieve good repeatability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
[0044] The present invention will be described in detail below using examples. However, the present invention is not limited to the forms shown in the examples, and the specific embodiments can be variously changed within the range described in the specific embodiments of the present invention.
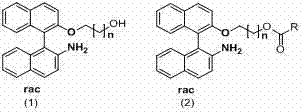
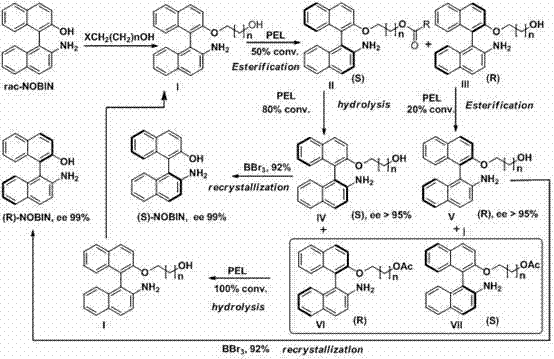
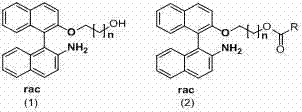
[0045] Preparation of racemic NOBIN and its derivatives
[0046] Preparation of racemic 2-amino-2'-hydroxy-1,1'-binaphthyl (NOBIN)
[0047] Add 20g of 2-naphthol and 20g of 2-naphthylamine into a 500mL round-bottomed flask, add 200mL of toluene under a nitrogen atmosphere, heat to reflux for 30min, cool and filter to obtain 34g of eutectic, with a yield of 85%. Dissolve 8g of eutectic into 150ml of water, add 30g of FeCl3.6H2O, and raise the temperature to 55°C for 4-8h under the protection of nitrogen. The reaction solution was cooled to room temperature and filtered. The filter cake was washed with distilled water, dried, passed through an activated carbon column, and eluted w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com