Preparation method and new application of artemisinin B
A compound and volume technology that can be used in antipyretics, organic chemistry, non-central analgesics, etc., to solve problems such as complex immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0025] 1. Experimental materials
[0026] 1.1 Animals: Female Wistar rats aged 8-10 weeks.
[0027] 1.2 Main reagents: Ficoll-Paque (Amersham Biosciences), LPS (E.coli0111:B4; Sigma; 1ng / mL), ELISA kit (R&D Systems, Minneapolis, MN). Medium: RPMI-1640 medium (Sigma-Aldrich), containing 10% calf serum, 2m ML-glutamine, 50μg / ml gentamicin and 5 damycin -5 M2-Mercaptoethanol (purchased from Sigma), the medium is freshly prepared before cell culture, 37 cells culture, 5% CO 2 to cultivate.
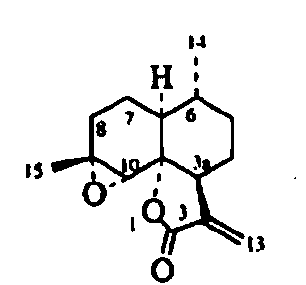
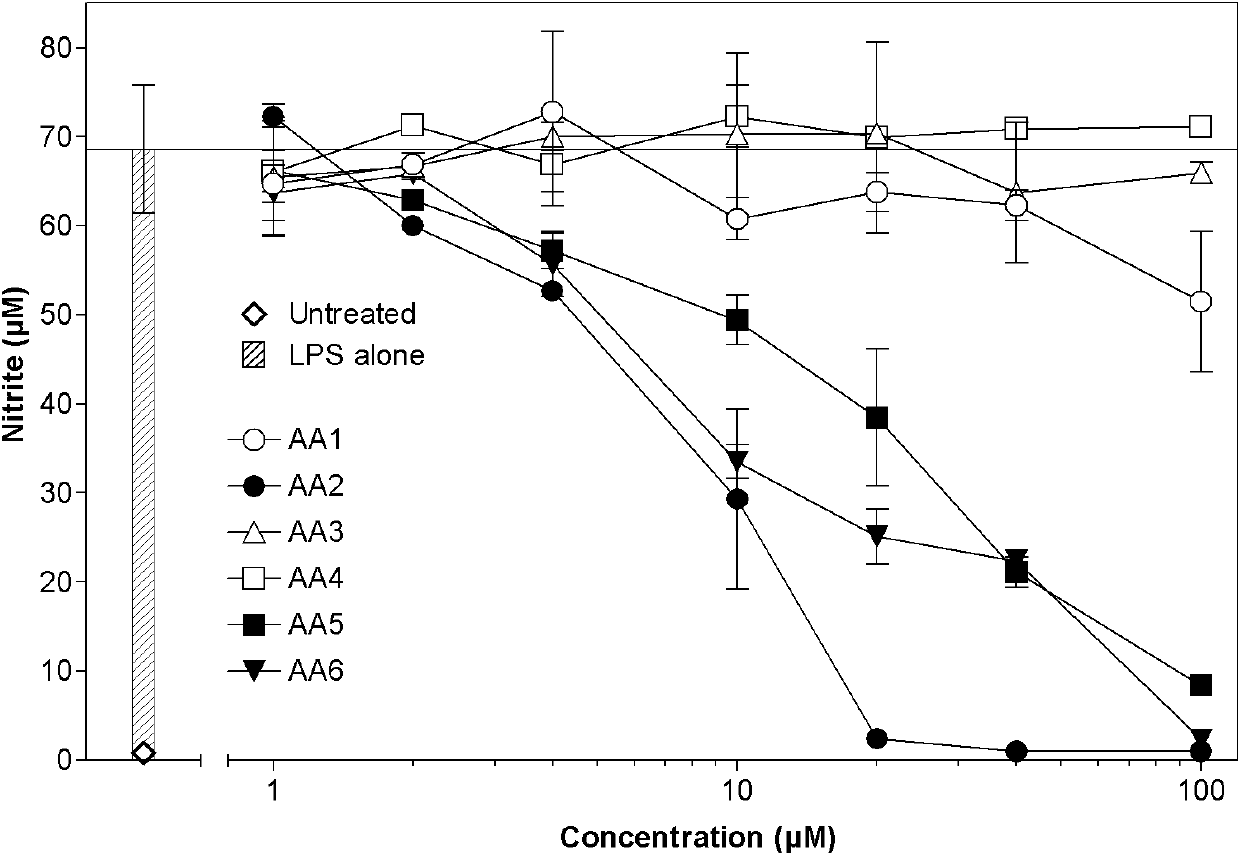
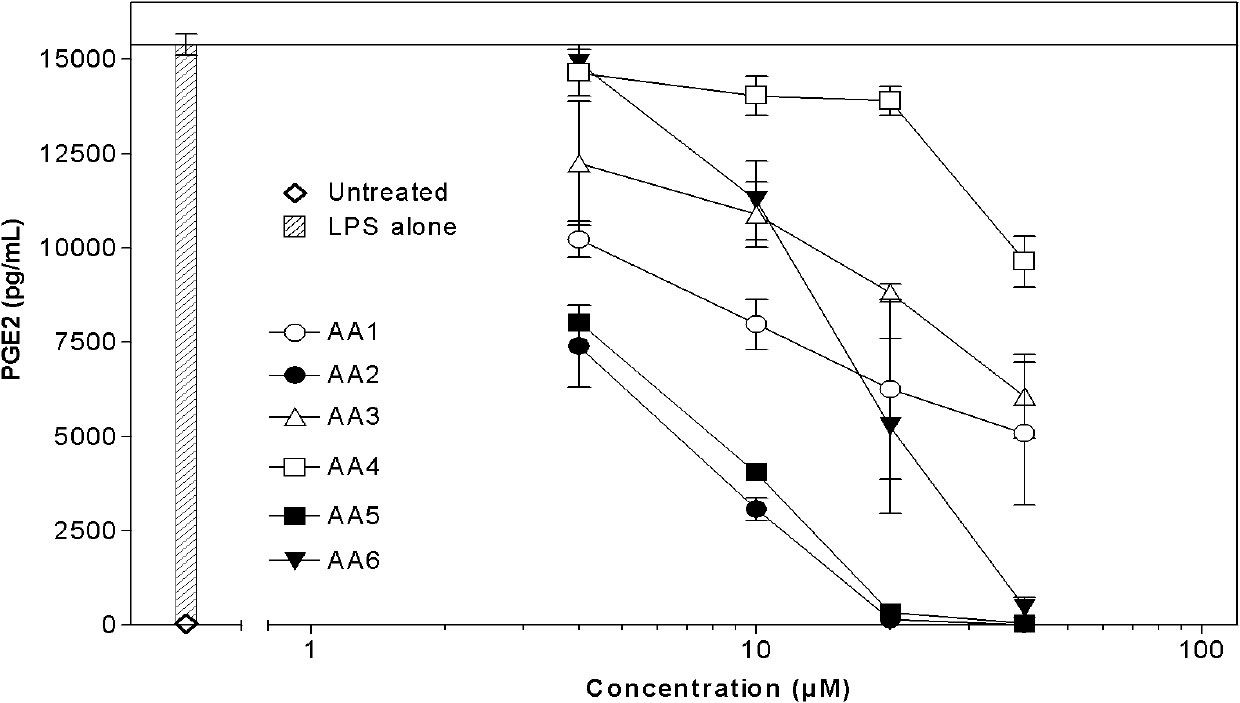
[0028] 1.3 The test drug is a series of compounds AA1-AA7 extracted from Artemisia annua L., where AA2 is artemisinin B.
[0029] 2. Experimental method
[0030] 2.1 Obtaining of rat abdominal cavity cells: 5-10 rats in a cage (transparent plastic mouse cage), fed in a standard animal room. Light: 6 to 18 o'clock; temperature: 22-24 ℃. After the vertebrae were sacrificed, 16ml of normal saline was injected into the abdominal cavity to wash the abdominal cavity cells with a concentration of 2×10 6 100...
Embodiment 1
[0052] After 1kg of Artemisia annua leaves are dried, crushed, add 5L of 95% ethanol to soak for 24 hours, percolate with 50L of 95% ethanol, collect the percolate, and recover the solvent under reduced pressure below 50℃ to obtain the extract. Mix the column chromatography silica gel with the weight of the extract, dry it, add it to the top of the column chromatography silica gel with 30 times the weight of the extract, use petroleum ether-ethyl acetate (1:0→5:5) Gradient elution, the components of the eluent were detected by thin-layer chromatography, the fractions containing artemisinin B were collected, the solvent was recovered to a small volume, and the crude crystals were separated out, and then purified by acetone recrystallization to obtain artemisinin B .
Embodiment 2
[0054] After 1kg of Artemisia annua leaves are dried, crushed, soaked in 4L of ethyl acetate for 50 hours, percolated with 45L of ethyl acetate, collected the percolate, and recovered the solvent under reduced pressure below 55°C to obtain the extract. After dissolving with ethyl acetate, Mix with column chromatography silica gel of 3 times the weight of the extract, dry, and add 25 times the weight of the extract to the top of the column chromatography silica gel for silica gel column chromatography. Use petroleum ether-ethyl acetate (1: 0→5:5) Gradient elution of mixed solvents, the components of the eluent are detected by thin layer chromatography, the fractions containing artemisinin B are collected, the solvent is recovered to a small volume, the crude crystals are precipitated out, and then subjected to ethyl acetate Repeated recrystallization and purification, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com