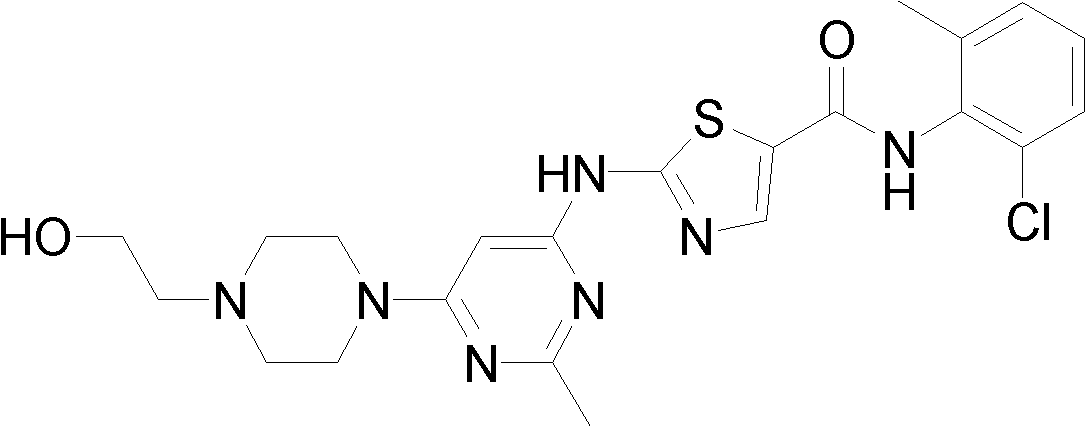
Method for preparing large particle size dasatinib
A technology of dasatinib and large particle size, which is applied in the field of preparing large particle size dasatinib, can solve the problems of increased preparation cost, large loss, long time consuming for filtration and washing treatment, etc., and achieves lower production cost and better flow rate Compressibility and compressibility, the effect of moderate solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 10 g of dasatinib and 300 ml of absolute ethanol to a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a thermometer to obtain a slurry; heat and reflux to 75° C. to obtain a clear solution; then, slowly cool the solution to 60 °C to obtain a suspension; then slowly cool down to room temperature for 10 hours, filter, and dry the obtained solid at 50 °C to obtain 8.9 g of a dry product. The particle size is: D (0.1) = 3.4 μm , D(0.5)=20.1 μm, D(0.9)=130.2 μm.
Embodiment 2
[0034] Add 10 g of dasatinib and 300 ml of absolute ethanol to a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a thermometer to obtain a slurry; heat and reflux to 75° C. to obtain a clear solution; then, slowly cool the solution to 60 °C to obtain a suspension; heat the suspension to 60-65 °C, then cool to 55 °C; keep warm at 55 °C and stir for 1 hour; heat to 65 °C again; slowly cool down to room temperature after 4 hours, filter, and The obtained solid was dried at 50° C. to obtain 9.1 g of a dry product, and its particle size was found to be: D(0.1)=6.4 μm, D(0.5)=55.2 μm, D(0.9)=140.3 μm.
Embodiment 3
[0036] Add 20 g of dasatinib and 700 ml of acetone to a 1000 ml four-necked flask equipped with a mechanical stirrer, a condenser and a thermometer to obtain a slurry; heat to reflux to 56° C. to obtain a clear solution; then, slowly cool the solution to 48° C. The suspension was obtained, and then slowly cooled to room temperature after 5 hours, the solution was filtered, and the obtained solid was dried at 50° C. to obtain 18.5 g of a dry product. The particle size was determined to be: D(0.1)=4.2 μm, D(0.5)=50.1 μm, D(0.9)=137.1 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com