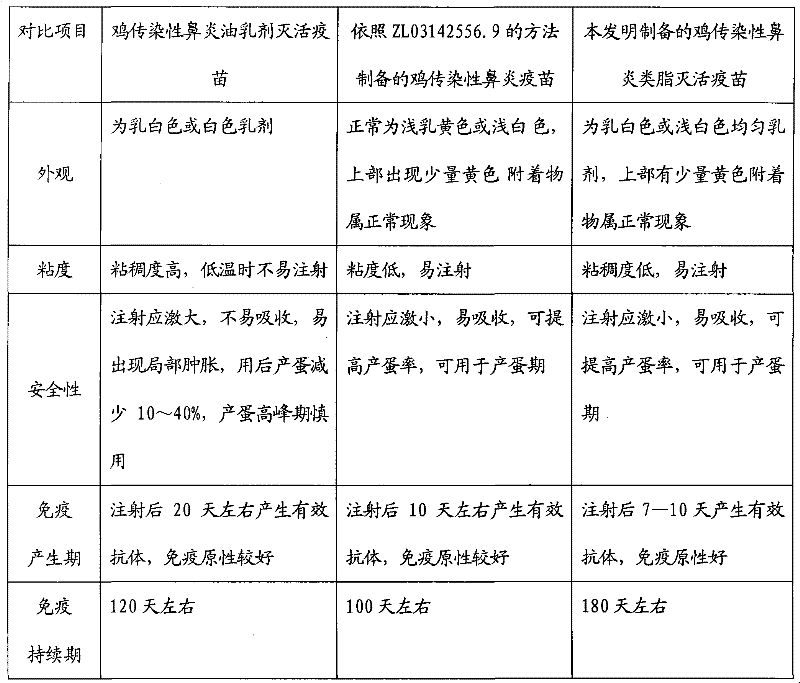
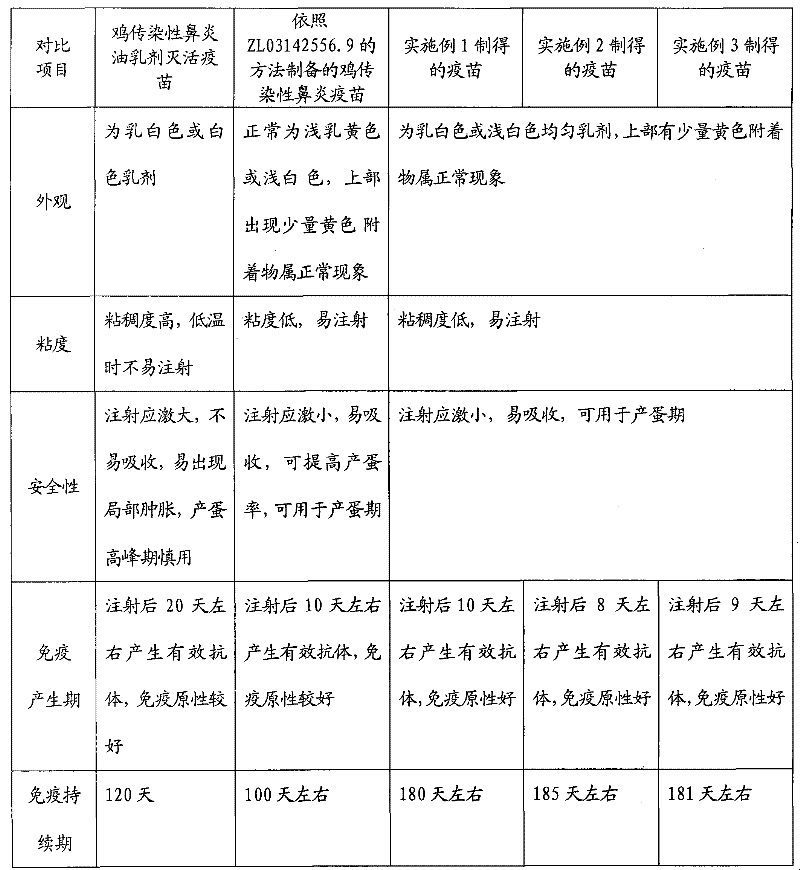
Preparation method of chicken infectious rhinitis lipid inactivated vaccine
A chicken infectious rhinitis and inactivated vaccine technology, applied in the field of preparation of veterinary biological products, to achieve the effects of saving production costs, increasing egg production rate, and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the preparation of chicken infectious rhinitis lipid inactivated vaccine, carries out according to the following steps:
[0027] (1) The preparation of type A Haemophilus paragallinarum inactivated antigen bacterium liquid is carried out in the following order:
[0028] Preparation of primary strains: Streak inoculation of Haemophilus paragallinarum type A strains on chicken broth agar plates, in CO 2 The volume content is 5%, the temperature is 16h in the candle jar of 37 ℃, select the 3 typical colonies of 1mm in diameter that meet the characteristics of the strain, dilute it with 10ml sterilized chicken broth and inoculate it into the yolk sac of 7-day-old SPF chicken embryo. Inoculate 0.2ml per embryo, continue to hatch at 37°C, collect the yolk fluid of dead embryos within 30 hours, and after pure inspection confirms that there is no miscellaneous bacteria, it will be used as a first-class strain and stored below -20°C for no more than 30 days ;
[...
Embodiment 2
[0040] Embodiment 2, the preparation of chicken infectious rhinitis lipid inactivated vaccine, carries out according to the following steps:
[0041] (1) The preparation of type A Haemophilus paragallinarum inactivated antigen bacterium liquid is carried out in the following order:
[0042] Preparation of primary strains: Streak inoculation of Haemophilus paragallinarum type A strains on chicken broth agar plates, in CO 2 The volume content is 5%, and the temperature is 17h in the candle jar of 37 ℃, selects 5 typical colonies with a diameter of 1mm that meet the characteristics of the strain, dilutes it with 10ml sterilized chicken broth, and inoculates it into the yolk sac of 6-day-old SPF chicken embryos. Embryos are inoculated with 0.2ml, continue to hatch at 37°C, collect the yolk fluid of dead embryos within 30 hours, and after pure inspection confirms that there is no miscellaneous bacteria, it will be used as a first-class strain and stored below -20°C for no more than...
Embodiment 3
[0049] Embodiment 3, the preparation of chicken infectious rhinitis lipid inactivated vaccine, carries out according to the following steps:
[0050] (1) The preparation of type A Haemophilus paragallinarum inactivated antigen bacterium liquid is carried out in the following order:
[0051] Preparation of primary strains: Streak inoculation of Haemophilus paragallinarum type A strains on chicken broth agar plates, in CO 2 The volume content is 5% and the temperature is 37 ° C for 18 hours. Select 3 typical colonies with a diameter of 1 mm that meet the characteristics of the strain and dilute them with 10 ml of sterilized chicken broth and inoculate them into the yolk sac of 7-day-old SPF chicken embryos. Each embryo is inoculated 0.2ml, continue to incubate at 37°C, collect the yolk fluid of dead embryos within 30 hours, and after pure inspection confirms that there are no bacteria, it will be used as a first-class strain and stored below -20°C for no more than 30 days;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com