Application of glucal and glucal derivative in preparation of drugs
A technology of gluculose and medicine is applied in the application field of gluculose and its derivatives in the preparation of medicines, and can solve problems such as unreported applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
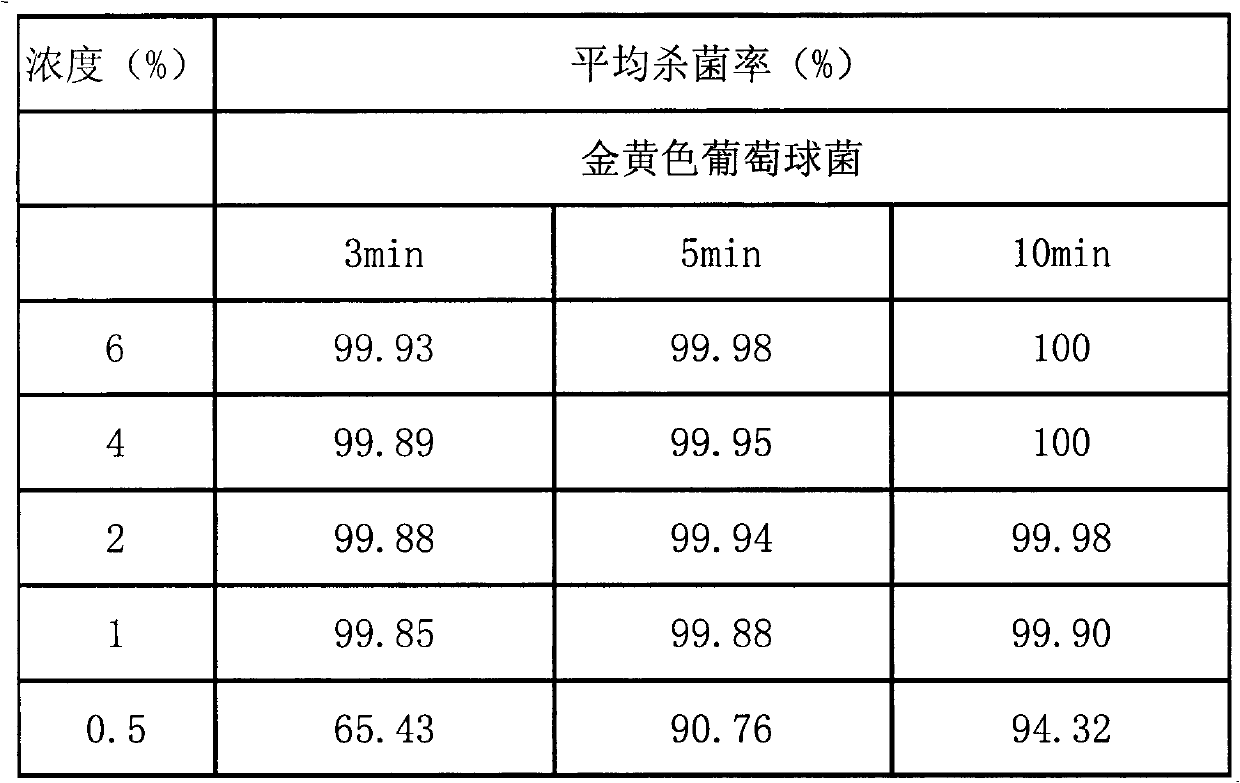
[0022] D-glucal antibacterial test:
[0023] Take the stock solution prepared by D-glucal 5g and 20mL propylene glycol, shake it well, make it even, and set aside. According to aseptic operation, take 8 bottles numbered A-F, and use the doubling dilution method to dilute the concentration to 1 / 2, 1 / 4, 1 / 8, 1 / 16, 1 / 32 of the stock solution concentration, and make a blank control. As follows: A: 15mL stock solution + 15mL propylene glycol, add 1mL of the remaining solution to 6 test tubes; B: 15mLA + 15mL propylene glycol, add 1mL of the remaining solution to 6 test tubes; C: 15mLB + 15mL propylene glycol, Add 1mL of the remaining solution to 6 test tubes; D: 15mL LC + 15mL propylene glycol, add 1mL of the remaining solution to 6 test tubes; E: 15mL D + 15mL propylene glycol, add 1mL of the remaining solution to 6 test tubes In a test tube; F: 15mL LE + 15mL propylene glycol, add 1 mL of the remaining solution to 6 test tubes; G: 3 mL propylene glycol, add 1 mL to 2 test tubes;...
Embodiment 2
[0029] Anti-inflammatory test of D-glucose mice:
[0030] The effect on mouse toe swelling and the effect of PGE2 exudation in inflammatory tissue (expressed as OD value)
[0031] Note; PGE2 prostaglandin E2 model: mouse paw egg white-induced inflammation method
[0032] test results:
[0033]
[0034] The test results in the table above show that D-glucal has a significant effect in inhibiting egg white-induced toe swelling, and is superior to hydrocortisone at high concentrations and doses. At the same time, it can effectively inhibit the exudation of PGE2 in toe swelling caused by egg white, and the effect is better than hydrocortisone
Embodiment 3
[0036] D-glucose antiviral test:
[0037] The 50% effective concentration (EC50) was calculated by Reed-Mucnch method and the therapeutic index IT=(using CC50 / EC50), the higher the IT value, the stronger the effect.
[0038] Virus strain:
[0039] Influenza virus A3, parainfluenza virus HVJ, adenovirus type 3, 7 (AdV3, 7), herpes simplex virus type I, II (HSVI, II)
[0040] Effect of D-glucal on cytopathic effect of virus
[0041] Virus
[0042] The results of in vitro antiviral tests show that D-glucal has different degrees of inhibitory effects on the above six viruses.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com