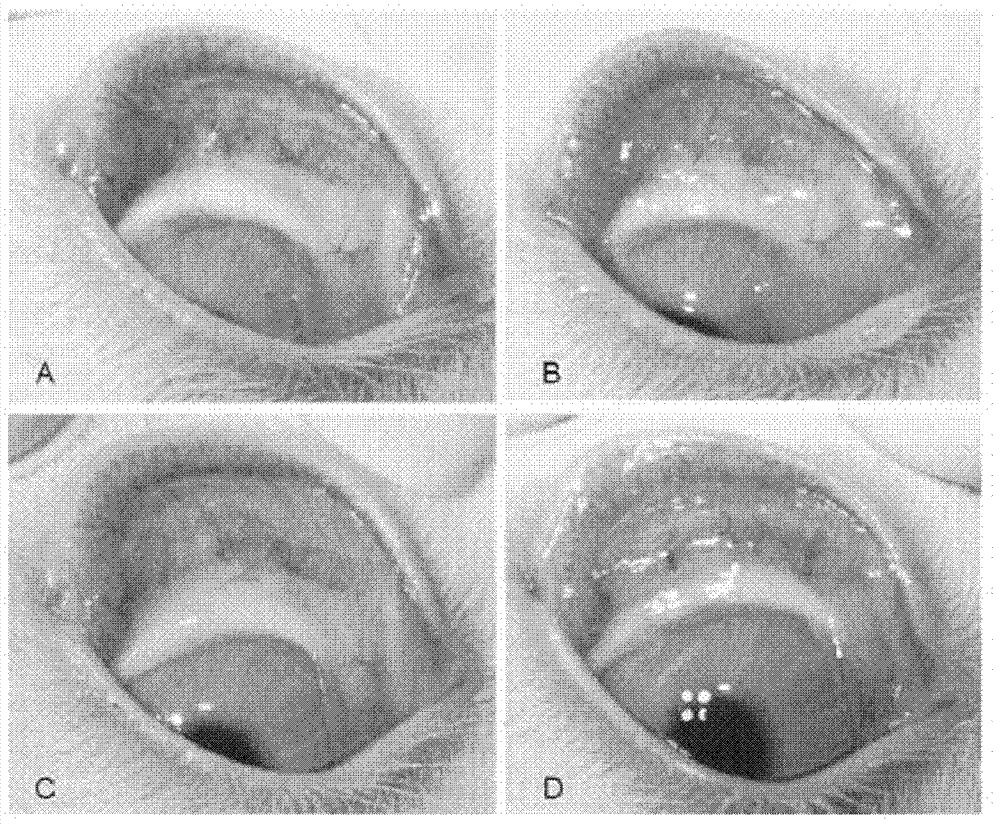
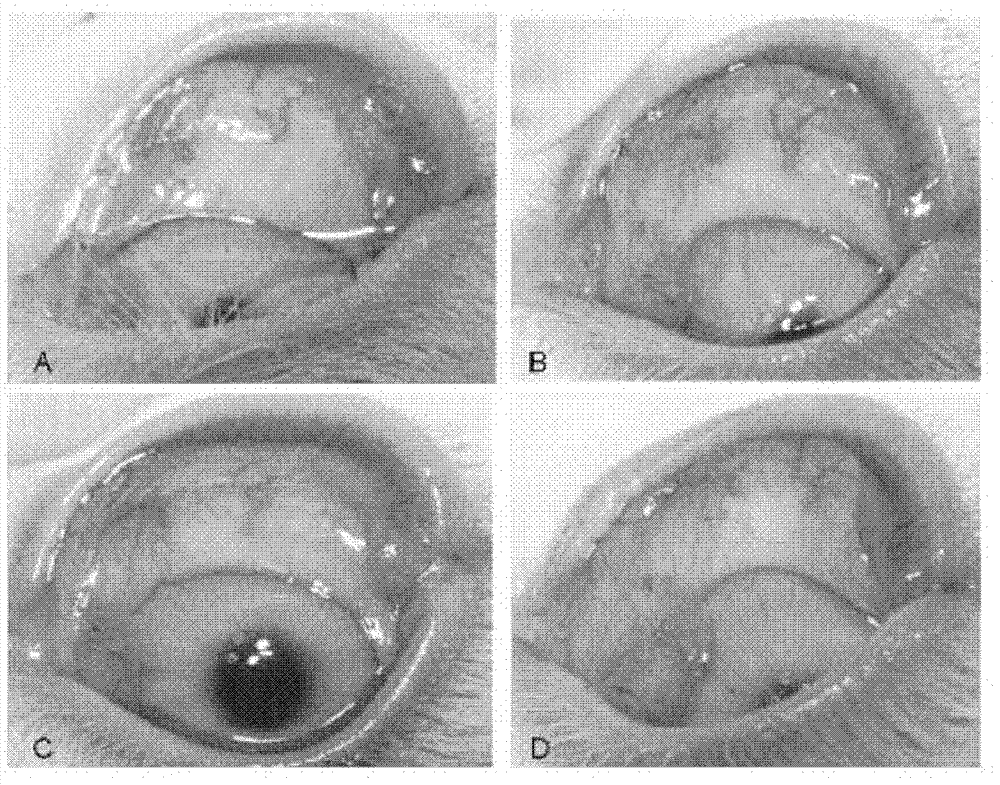
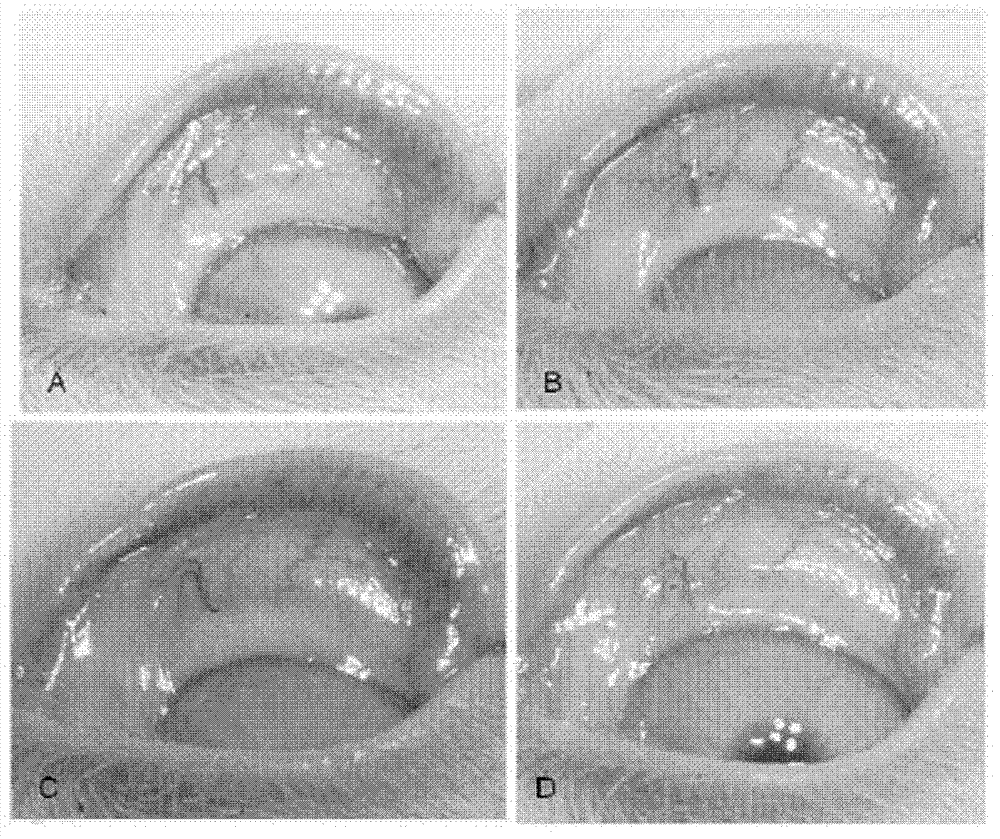
Application of pirfenidone in preparation of medicaments for controlling proliferative diseases after ophthalmologic operation and eye drops thereof
A technology of pirfenidone and cataract, which is applied in the application of medicine and the field of eye drops, can solve the problems of patients' vision occlusion, affecting the visual function recovery of cataract patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Each 100ml of pirfenidone eye drops in this example is prepared as follows: 0.1g of pirfenidone is moistened with a small amount of water for injection, and then dissolved with 3ml of 10% (volume fraction) acetic acid aqueous solution, and water for injection is added Adjust the pH to about 80ml with 1N NaOH, then add 0.8g sodium chloride, 0.2g sodium citrate, 0.002g thimerosal, adjust to pH=7, add sterile water for injection to 100ml, filter 0.22μm , Aseptic packaging.
Embodiment 2
[0187] Each 100ml of pirfenidone eye drops in this example is prepared as follows: 0.5g of pirfenidone is moistened with a small amount of water for injection, and then dissolved with 4ml of 10% (volume fraction) acetic acid aqueous solution, and water for injection is added Adjust the pH to about 80ml with 1N NaOH, then add 0.75g sodium chloride, 0.2g sodium citrate, 0.002g thimerosal, adjust to pH=7, add sterile water for injection to 100ml, filter 0.22μm , Aseptic packaging.
Embodiment 3
[0189] Each 100ml of pirfenidone eye drops in this example is prepared as follows: 1g of pirfenidone is moistened with a small amount of water for injection, and then dissolved with 5ml of 10% (volume fraction) acetic acid aqueous solution, and water for injection is added to About 80ml, adjust the pH to about 6 with 1N NaOH, then add 0.7g of sodium chloride, 0.2g of sodium citrate, 0.002g of thimerosal, adjust to pH=7, add sterile water for injection to 100ml, filter with 0.22μm, Aseptic packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com