Synthesis method of halogen acetone
A synthesis method, acetone technology, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as high price of auxiliary catalysts, strong acidity of trifluoroacetic acid, increased synthesis costs, etc., and achieve operating costs Low, high selectivity, high productivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
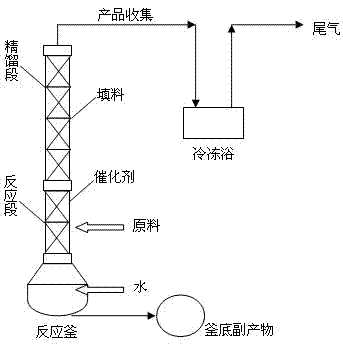
[0025] The synthetic method step of halogenated acetone is as follows:
[0026] The synthesis method of halogenated acetone adopts a reactive distillation device, the height ratio of the reaction section of the reactive distillation device to the rectification section is 1:3 to 1:6, and 0.1 equivalent to 2 equivalents of water is added to the bottom of the reactive distillation device, and heated to 95 ℃ ~ 100 ℃, add ethyl haloacetoacetate in the middle of the reaction section, the molar ratio of ethyl haloacetoacetate to water is 1:1 ~ 1:2.5, and fill the reaction section with strong acidic cation exchange resin as a catalyst , ethyl haloacetoacetate reacts with water vapor after being catalyzed by the reaction section, and is separated by the rectification section. The product haloacetone is collected at the top of the rectification section, and the product collection bottle is placed in a freezing bath at -10°C to -60°C. The collected product was greater than 99% pure.
[...
Embodiment 1
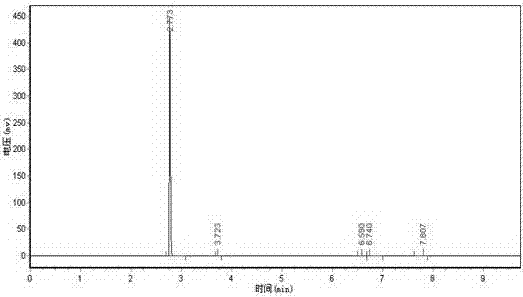
[0036] Design the tower tank as a 20L reactor, the height ratio of the reaction section to the rectification section is 1:6, add 0.1 equivalent of water to the bottom of the reaction distillation device, heat to 100°C, add ethyl trifluoroacetoacetate in the middle of the reaction section, three The molar ratio of ethyl fluoroacetoacetate to water is 1:2.5. The reaction section is filled with strong acidic cation exchange resin NKC-9. After being catalyzed in the reaction section, ethyl trifluoroacetoacetate reacts with water vapor and is separated by the rectification section. , the product trifluoroacetone was collected at the top of the rectification section, and the product collection bottle was placed in a -60°C freezing bath. The collected product had a purity of 99.5% and a yield of 95%.
Embodiment 2
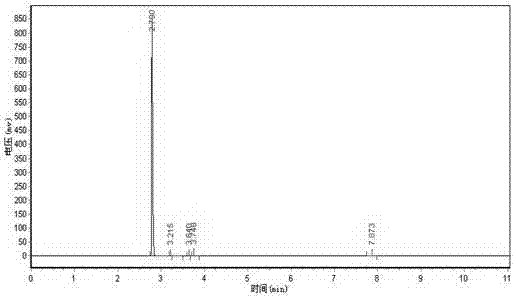
[0038] Design the tower kettle as a 20L reactor, the height ratio of the reaction section to the rectification section is 1:3, add 2 equivalents of water to the bottom of the reaction distillation device, heat to 100°C, add ethyl trifluoroacetoacetate in the middle of the reaction section, three The molar ratio of ethyl fluoroacetoacetate to water is 1:1. The reaction section is filled with strong acidic cation exchange resin NKC-9. After being catalyzed in the reaction section, ethyl trifluoroacetoacetate reacts with water vapor and is separated by the rectification section. , the product trifluoroacetone was collected at the top of the rectification section, and the product collection bottle was placed in a -40°C freezing bath. The collected product had a purity of 99.2% and a yield of 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com