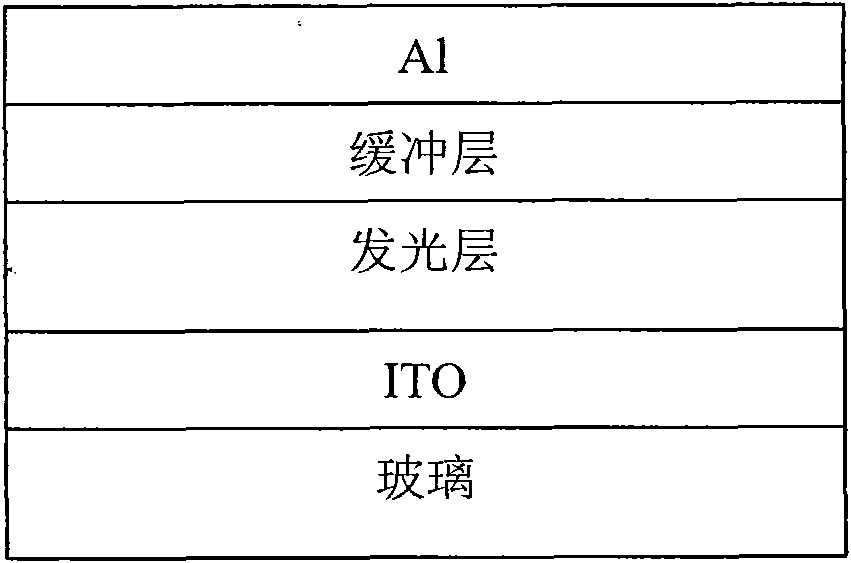
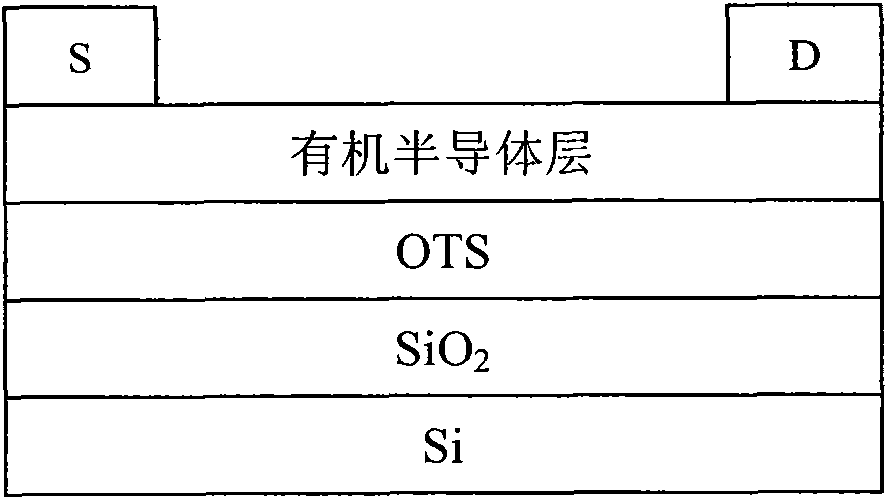
Conjugated polymer containing cyclopentadienyl diene dithiophene-naphthalene tetracarboxylic diimide and preparation method and application thereof
A technology of naphthalene tetracarboxylic acid diimide and cyclopentadiene dithiophene, which is applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc., and can solve the mismatch between the spectral response of the device and the solar radiation spectrum , the red light region has not been effectively utilized, low carrier mobility and other problems, to achieve the effect of high carrier mobility, high molar absorptivity, and wide light absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of cyclopentadiene-containing dithiophene-naphthalene tetracarboxylic diimide conjugated polymer (n=35, x=1, y=1)
[0052]
[0053] Under the condition of nitrogen protection, compound N, N'-dioctyl-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide (0.32g, 0.5mmol) and 4 , 4-bis(eicosyl)-2,6-bistrimethyltin-cyclopentadiene (2,1-b:3,4-b')dithiophene (0.53g, 0.5mmol) chloride Benzene (25mL) solution was bubbled for 0.5h to remove residual oxygen, and then Pd was added 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3 (0.0083g, 0.027mmol), and bubbling for 1h to remove residual oxygen, then heated to 30°C and refluxed for 72h.
[0054] Add the mixed liquid dropwise to methanol for sedimentation, and dissolve it with chlorobenzene after suction filtration, methanol washing and drying steps, add it to the aqueous solution of sodium diethyldithiocarbamate, heat to 80°C and stir overnight. Pass the organic phase through alumina column chromat...
Embodiment 2
[0055] Example 2 Preparation of cyclopentadiene-containing dithiophene-naphthalene tetracarboxylic acid diimide conjugated polymer (n=71, x=1, y=1)
[0056]
[0057] Under argon protection condition, to containing compound N, N'-two-(n-eicosyl)-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic diimide (0.49 g, 0.5mmol) and 4,4-dioctyl-2,6-bistrimethyltin-cyclopentadiene (2,1-b:3,4-b')dithiophene (0.36g, 0.5mmol ) in chlorobenzene (25mL) solution bubbled for 0.5h to remove residual oxygen, then added Pd 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3(0.0083g, 0.027mmol), and bubbling for 1h to remove residual oxygen, then heated to 60°C for 24h under reflux.
[0058] Add the mixed liquid dropwise to methanol for sedimentation, and dissolve it with chlorobenzene after suction filtration, methanol washing and drying steps, add it to the aqueous solution of sodium diethyldithiocarbamate, heat to 80°C and stir overnight. Pass the organic phase through alumina column chromatograph...
Embodiment 3
[0059] Example 3 Preparation of cyclopentadiene-containing dithiophene-naphthalene tetracarboxylic acid diimide conjugated polymer (n=57, x=1, y=1)
[0060]
[0061] Under nitrogen protection conditions, the compound N, N'-bis-(1-octylnonyl)-2,6-dibromo-1,4,5,8-naphthalene tetracarboxylic acid diimide (0.45g , 0.5mmol) and 4,4-dioctyl-2,6-bistrimethyltin-cyclopentadiene (2,1-b:3,4-b')dithiophene (0.36g, 0.5mmol) Chlorobenzene (25mL) solution was bubbled for 0.5h to remove residual oxygen, then added Pd 2 (dba) 3 (0.014g, 0.015mmol) and P(o-Tol) 3 (0.0083g, 0.027mmol), and bubbling for 1h to remove residual oxygen, then heated to 80°C for 48h under reflux.
[0062] Add the mixed solution dropwise to methanol for sedimentation, and dissolve it with chlorobenzene after suction filtration, methanol washing and drying steps, add it to the aqueous solution of sodium diethyldithiocarbamate, heat to 90°C and stir overnight. Pass the organic phase through alumina column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com