Method for synthesizing Ramelteon
A technology of ramelteon and ramelteon, applied in the field of ramelteon synthesis, can solve problems such as loss of advantages, and achieve the effects of reducing process cost, being beneficial to environmental protection and having high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
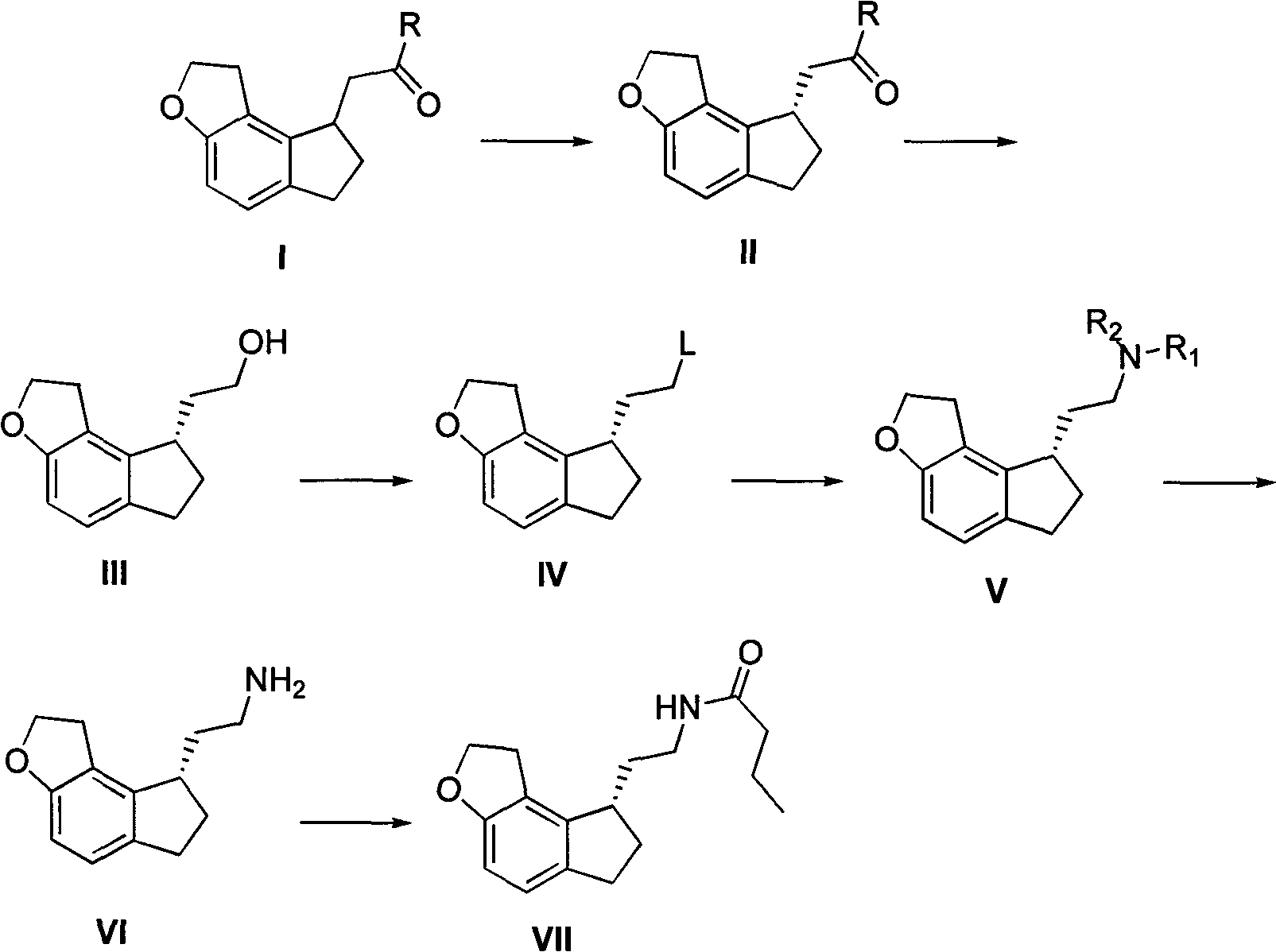
[0034] Example 1: Add 10 g of ramecic acid mixture I (MW: 218, 0.0459 mol), add 60 ml of ethanol, dropwise add 4 ml of thionyl chloride (MW: 119, 0.0551 mol), and react at room temperature until the raw materials disappear After evaporating ethanol under reduced pressure, add 60ml of ethyl acetate and slowly add 60ml of saturated aqueous solution of sodium bicarbonate dropwise, adjust the pH to neutral, separate layers, extract the aqueous phase with 30ml of ethyl acetate, combine the organic phases, anhydrous Dry over sodium sulfate, evaporate ethyl acetate under reduced pressure, and dry under high vacuum to obtain 11.2 g of ethyl ramelate I. The product purity is greater than 99%, and the yield is quantitative. Chiral separation can be performed directly without purification.
[0035] 2. Synthesis of Ramelyl Alcohol III
[0036] Example 1: The crude product obtained in the example of ethyl ramelate I was separated by a chiral column to obtain 5.5 g (MW: 246, 22.36 mmol) of ...
Embodiment 2
[0037] Example 2: 16.5 g (MW: 246, 67.07 mmol) of chiral ethyl ramacate II obtained after the crude product obtained in the example of ethyl ramaceate I was separated by a chiral column was mixed in 66 ml of THF, and NaBH was added 4 5.1g (MW: 38, 0.134mol), N, N-dimethylaniline 8.5ml (MW: 121, 67.4mmol), ZnCl 2 9.2g (MW: 136, 67.65mmol), heated to reflux until the raw materials disappeared, evaporated THF to dryness under reduced pressure, added 50ml of 2M HCl, 100ml of DCM and stirred for 30min, then separated the organic layer, and washed the organic layer with 2M HCl until no N, N -Dimethylaniline, the organic phase was dried, filtered, and evaporated to dryness to obtain 13.2 g of ramelol III, with a yield of 96.5% and a purity greater than 97%, which can be directly used for the next step. NaBH here 4 The molar ratio with chiral ethyl ramanate II can be controlled in the range of 3-1.
[0038] 3. Synthesis of compound IV ramemesylate
[0039] Example 1: Take 4.6g (M...
Embodiment 3
[0044]Example 3: Take 3 g of bromide of ramelyl III (FW: 267, 0.0112 mol), mix 0.9 g of sodium azide (FW: 66, 0.0136 mol) in 10 ml of DMF, heat to 80 degrees and stir until TLC detects the raw material disappear, add 100ml of water and 150ml of ethyl acetate to the system, stir and extract by layers, back extract the aqueous phase once with 100ml of ethyl acetate, combine the organic phases, wash three times with 100ml of saturated brine, evaporate the organic layer to dryness, and dissolve the crude product in Add 10 mg of Pd / C to 10 ml of methanol and 70 ml of ethyl acetate, catalyze hydrogenation, stir at room temperature until TLC detects that the raw material disappears, filter the system to remove Pd / C, and directly evaporate the filtrate to obtain the crude product of ramelamine VI, which becomes a salt Salt, the method is the same as above, finally get the hydrochloride 2.6g of ramelamine, yield 96.6%.
[0045] 5. Synthesis of Ramelteon VII
[0046] Example 1: Take 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com