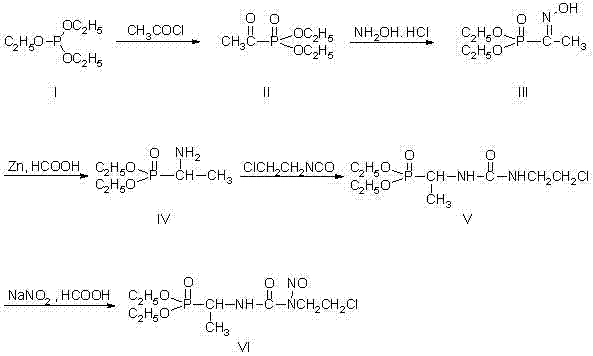Synthetic method of fotemustine bulk drug
A synthesis method and fomustine technology, applied in the field of medicinal chemistry, can solve the problems of restricting domestic manufacturers' technological development and production, difficulty in domestic purchase of raw materials, and high production costs, and achieve simple production steps, good product quality, and simple control. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of formustine specifically adopts the following steps:
[0029] (1) Synthesis of compound (II):
[0030] 73.2g (0.44mol) of triethyl phosphite was added to a 250mL dry reaction flask, 31.4g (0.40mol) of acetyl chloride was added dropwise, and the reaction was stirred at room temperature for 12h. Distilled under reduced pressure and collected fractions at 80-84°C / 3mmHg to obtain 58.4g of compound (II) with a yield of 81%.
[0031] (2) Synthesis of compound (Ⅲ):
[0032] In a 500mL reaction bottle, add 32g of hydroxylamine hydrochloride and 70mL of water. Add sodium hydroxide solution (18.2g sodium hydroxide dissolved in 50mL water) dropwise under stirring, control the temperature between 20~25°C, add 56g compound (II) dropwise, and stir at room temperature for 12h. The reaction mixture was extracted with 100 mL×3 dichloromethane, and the organic layer was collected and dried by adding anhydrous magnesium sulfate. After filtration, the solvent was...
Embodiment 2
[0040] The synthetic method of formustine specifically adopts the following steps:
[0041] (1) Synthesis of compound (II):
[0042] Add 73.2g (0.44mol) of triethyl phosphite to a 250mL dry reaction flask, control the temperature between 20 and 30°C, and add 31.4g (0.40mol) of acetyl chloride dropwise. The temperature was maintained and the reaction was stirred for 12h. Distilled under reduced pressure to remove the lower boiling fraction (<40°C) to obtain 78 g of colorless liquid, which is the crude product of compound (II).
[0043] (2) Synthesis of compound (Ⅲ):
[0044] In a 500mL reaction flask, add 32g of hydroxylamine hydrochloride and 125mL of water. Control the temperature between -10 and 0°C, add 37g of sodium bicarbonate in batches, and then dropwise to obtain 78g of crude compound (II) as a colorless liquid, and stir and react at room temperature for 24h. The reaction mixture was extracted with 100 mL×3 dichloromethane, the organic phases were combined, washed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com